Balbharti Maharashtra State Board 12th Chemistry Textbook Solutions
Chapter 7 Elements of Groups 16, 17 and 18 Textbook Exercise Questions and Answers.
1. Select appropriate answers for the following.
Question i.
Which of the following has the highest electron gain enthalpy?
A. Fluorine
B. Chlorine
C. Bromine
D. Iodine
Answer:
B. Chlorine

Question ii.
Hydrides of group 16 are weakly acidic. The correct order of acidity is
A. H2O > H2S > H2Se > H2Te
B. H2Te > H2O > H2S > H2Se
C. H2Te > H2Se > H2S > H2O
D. H2Te > H2Se > H2O > H2S
Answer:
C. H2Te > H2Se > H2S > H2O
Question iii.
Which of the following element does not show oxidation state of +4 ?
A. O
B. S
C. Se
D. Te
Answer:
A. O
Question iv.
HI acid when heated with conc. H2SO4 forms
A. HIO3
B. KIO3
C. I2
D. KI
Answer:
C. I2
Question v.
Ozone layer is depleted by
A. NO
B. NO2
C. NO3
D. N2O5
Answer:
A. NO
Question vi.
Which of the following occurs in liquid state at room temperature?
A. HIO3
B. HBr
C. HCl
D. HF
Answer:
D. HF
Question vii.
In pyrosulfurous acid oxidation state of sulfur is
A. Only +2
B. Only +4
C. +2 and +6
D. Only +6
Answer:
B. Only + 4
Question viii.
Stability of interhalogen compounds follows the order
A. BrF > IBr > ICl > ClF > BrCl
B. IBr > BeF > ICl > ClF > BrCl
C. ClF > ICl > IBr > BrCl > BrF
D. ICl > ClF > BrCl > IBr > BrF
Answer:
C. ClF > ICl > IBr > BrCl > BrF
Question ix.
BrCl reacts with water to form
A. HBr
B. Br2 + Cl2
C. HOBr
D. HOBr + HCl
Answer:
D. HOBr + HCl

Question x.
Chlorine reacts with excess of fluorine to form.
A. ClF
B. ClF3
C. ClF2
D. Cl2F3
Answer:
B. ClF3
Question xi.
In interhalogen compounds, which of the following halogens is never the central atom.
A. I
B. Cl
C. Br
D. F
Answer:
D. F
Question xii.
Which of the following has one lone pair of electrons?
A. IF3
B. ICl
C. IF5
D. ClF3
Answer:
C. IF5
Question xiii.
In which of the following pairs, molecules are paired with their correct shapes?
A. [I3] : bent
B. BrF5 : trigonal bipyramid
C. ClF3 : trigonal planar
D. [BrF4] : square planar
Answer:
A. [I3] : bent
Question xiv.
Among the known interhalogen compounds, the maximum number of atoms is
A. 3
B. 6
C. 7
D. 8
Answer:
D. 8
2. Answer the following.
Question i.
Write the order of the thermal stability of the hydrides of group 16 elements.
Answer:
The thermal stability of the hydrides of group 16 elements decreases in the order of H2O > H2S > H2Se > H2Te.
Question ii.
What is the oxidation state of Te in TeO2?
Answer:
The oxidation state of Te in TeO2 is + 4.
Question iii.
Name two gases which deplete ozone layer.
Answer:
Nitrogen oxide (NO) released from exhaust systems of car or supersonic jet aeroplanes and chlorofluorocarbons (Freons) used in aerosol sprays and refrigerators deplete ozone layer.
Question iv.
Give two uses of ClO2
Answer:
(i) ClO2 is used as a bleaching agent for paper pulp and textiles.
(ii) It is also used in water treatment.
Question v.
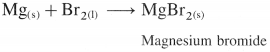
What is the action of bromine on magnesium metal?
Answer:
Bromine reacts instantly with magnesium metal to give magnesium bromide.

Question vi.
Write the names of allotropic forms of selenium.
Answer:
Selenium has two allotropic forms as follows :
(i) Red (non-metallic) form
(ii) Grey (metallic) form
Question vii.
What is the oxidation state of S in H2SO4.
Answer:
The oxidation state of S in H2SO4 is + 6.
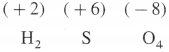
Question viii.
The pKa values of HCl is -7.0 and that of HI is -10.0. Which is the stronger acid?
Answer:
For HCl, pKa = -7.0, hence its dissoClation constant is, Ka = 1 x 10-7.
For HI pKa = – 10.0, hence its dissoClation constant is Ka = 1 x 10-7. Hence HCl dissoClates more than HI.
Therefore HCl is a stronger acid than HI.
Question ix.
Give one example showing reducing property of ozone.
Answer:
Ozone decomposes to liberate nascent oxygen, hence it is a powerful oxidising agent. O3(g) → O2(g) + O
For example :
(i) It oxidises lead sulphide (PbS) to lead sulphate (PbSO4).
pbS(s) + 4O3(g) → PbSO(s) + 4O2(g)
(ii) Potassium iodide, KI is oxidised to iodine, I2 in the solution.
2KI(aq) + H2O(1) + O3(g) → 2KOH(aq) + I2(s) + O2(g)
Question x.
Write the reaction of conc. H2SO4 with sugar.
Answer:
Concentrated sulphuric acid when added to sugar, it is dehydrated giving carbon.
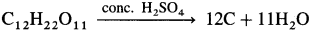
The carbon that is left behind is called sugar charcoal and the process is called char.
Question xi.
Give two uses of chlorine.
Answer:
Chlorine is used for :
Question xii.
Complete the following.
1. ICl3 + H2O …….. + …….. + ICl
2. I2 + KClO3 ……. + KIO2
3. BrCl + H2O ……. + HCl
4. Cl2 + ClF3 ……..
5. H2C = CH2 + ICl …….
6. XeF4 + SiO2 ……. + SiF4
7. XeF6 + 6H2O …….. + HF
8. XeOF4 + H2O ……. + HF
Answer:
1. 2ICI3 + 3H2O → 5HCl + HlO3 + ICl
2. I2 + KCIO3 → ICl + KIO3
3. BrCl + H2O → HOBr + HCl
4. Cl2 + C1F3 → 3ClF
5. CH2 = CH2 + ICl → CH2I – CH2Cl
6. 2XeF6 + SiO2 → 2XeOF4 + SiF4
7. XeF6 + 3H2O → XeO3 + 6HF
8. XeOF4 + H2O→ XeO2F2 + 2HF

Question xiii.
Match the following
A – B
XeOF2 – Xenon trioxydifluoride
XeO2F2 – Xenon monooxydifluoride
XeO3F2 – Xenon dioxytetrafluoride
XeO2F4 – Xenon dioxydifluoride
Answer:
XeOF2 – Xenon monooxydifluoride
XeO2F2 – Xenon dioxydifluoride
XeO3F2 – Xenon trioxydifluoride
XeO2F4 – Xenon dioxytetrafluoride
Question xiv.
What is the oxidation state of xenon in the following compounds?
XeOF4, XeO3, XeF5, XeF4, XeF2.
Answer:
3. Answer the following.
Question i.
The first ionisation enthalpies of S, Cl and Ar are 1000, 1256 and 1520 kJ/mol-1, respectively. Explain the observed trend.
Answer:
(i) The atomic number increases as, 16S < 17Cl < 18Ar1.
(ii) Due to decrease in atomic size and increase in effective nuclear charge, Cl binds valence electrons strongly.
(iii) Hence ionisation enthalpy of Cl (1256 kJ mol-1) is higher than that of S(1000 kJ mol-1)
(iv) Ar has electronic configuration 3s23p6. Since all electrons are paired and the octet is complete, it has the highest ionisation enthalpy, (1520 kJ mol-1)
Question ii.
“Acidic character of hydrides of group 16 elements increases from H2O to H2Te” Explain.
Answer:
(i) The thermal stability of the hydrides of group 16 elements decreases from H2O to H2Te. This is because the bond dissociation enthalpy of the H-E bond decreases down the group.
(ii) Thus, the acidic character increases from H2O to H2Te.
Question iii.
How is dioxygen prepared in laboratory from KClO3?
Answer:
By heating chlorates, nitrates and permanganates.
Potassium chlorate in the presence of manganese dioxide on heating decomposes to form potassium chloride and oxygen.
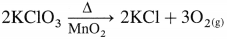
Question iv.
What happens when
a. Lead sulfide reacts with ozone (O3).
b. Nitric oxide reacts with ozone.
Answer:
(i) It oxidises lead sulphide (PbS) to lead sulphate (PbSO4) changing the oxidation state of S from – 2 to +6.
PbS(s) + 4O3(g) → PbSO(s) + 4O2(g)
(ii) Ozone oxidises nitrogen oxide to nitrogen dioxide.
NO(g) + O3(g) → NO2(g) + O2(g)
Question v.
Give two chemical reactions to explain oxidizing property of concentrated H2SO4.
Answer:
Hot and concentrated H2SO4 acts as an oxidising agent, since it gives nascent oxygen on heating.
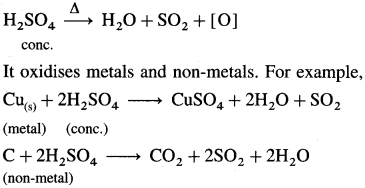

Question vi.
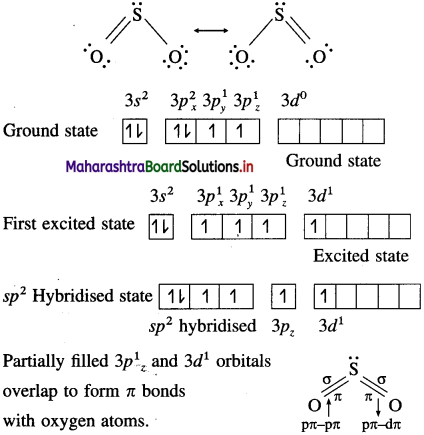
Discuss the structure of sulfur dioxide.
Answer:
(i) SO2 molecule has a bent V shaped structure with S-O-S bond angle 119.5° and bond dissoClation enthalpy is 297 kJ mol-1.
(ii) Sulphur in SO2 is sp2 hybridised forming three hybrid orbitals. Due to lone pair electrons, bond angle is reduced from 120° to 119.5°.
(iii) In SO2, each oxygen atom is bonded to sulphur by σ and a π bond.
(iv) a bond between S and O are formed by sp2-p overlapping.
(v) One of π bonds is formed by pπ – pπ overlapping while other n bond is formed by pπ – dπ overlap.
(vi) Due to resonance both the bonds are identical having observed bond length 143 pm due to resonance,
Question vii.
Fluorine shows only -1 oxidation state while other halogens show -1, +1, +3, +5 and +7 oxidation states. Explain.
Answer:
Question viii.
What is the action of chlorine on the following
a. Fe
b. Excess of NH3
Answer:
(a) Chlorine reacts with Fe to give ferric chloride.
2Fe + 3Cl2 → 2FeCl3
(b) Chlorine reacts with the excess of ammonia to form ammonium chloride, NH4Cl and nitrogen.

Question ix.
How is hydrogen chloride prepared from sodium chloride?
Answer:

Question x.
Draw structures of XeF6, XeO3, XeOF4, XeF2.
Answer:
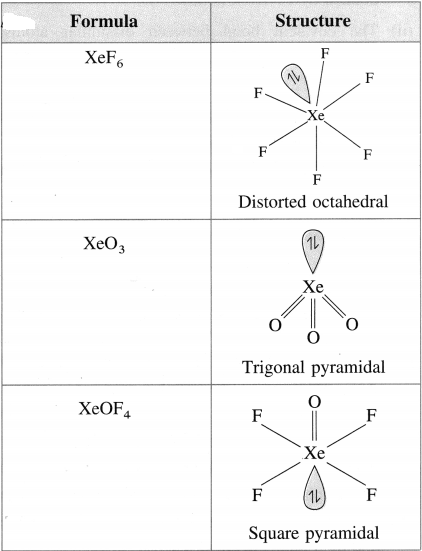
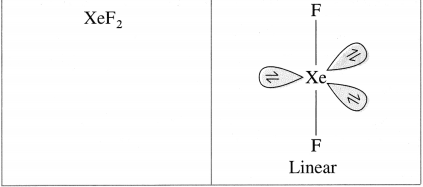
Question xi.
What are interhalogen compounds? Give two examples.
Answer:
Interhalogen compounds : Compounds formed by the combination of atoms of two different halogens are called interhalogen compounds. In an interhalogen compound, of the two halogen atoms, one atom is more electropositive than the other. The interhalogen compound is regarded as the halide of the more electropositive halogen.
For example ClF, BrF3, ICl
Question xii.
What is the action of hydrochloric acid on the following?
a. NH3
b. Na2CO3
Answer:
a. Hydrochloric acid reacts with ammonia to give white fumes of ammonium chloride.
NH3 + HCl → NH4Cl
b. Hydrochloric acid reacts with sodium carbonate to give sodium chloride, water with the liberation of carbon dioxide gas.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Question xiii.
Give two uses of HCl.
Answer:
Hydrogen chloride (OR hydrochloric acid) is used :
Question xiv.
Write the names and structural formulae of oxoacids of chlorine.
Answer:
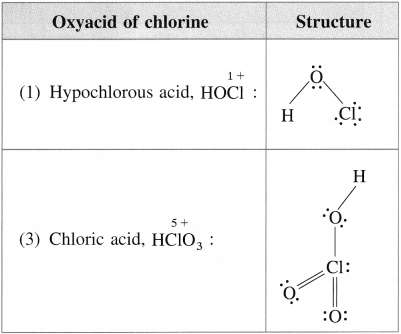
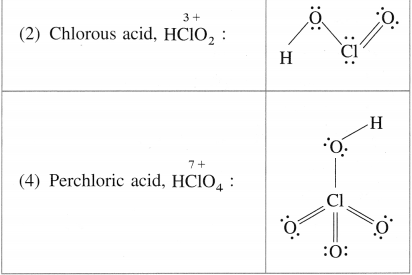

Question xv.
What happens when
a. Cl2 reacts with F2 in equal volume at 437 K.
b. Br2 reacts with excess of F2.
Answer:
(a) Cl2 reacts with F2 in equal volumes at 437 K to give chlorine monofluoride ClF.
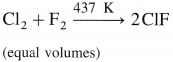
(b) Br2 reacts with excess of F2 to give bromine trifluoride BF3.
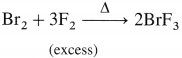
Question xvi.
How are xenon fluorides XeF2, XeF4 and XeF6 obtained ? Give suitable reactions.
Answer:
Xenon fluorides are generally prepared by the direct reaction of xenon and fluorine in different ratios and under appropriate experimental conditions, such as temperature, in the presence of an electric discharge and by a photochemical reaction.
(i) Preparation of XeF2 :
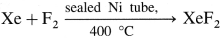
(ii) Preparation of XeF4 :

(iii) Preparation of XeF6 :

Question xvii.
How are XeO3 and XeOF4 prepared?
Answer:
Preparation of XeO3 : Xenon trioxide (XeO3) is prepared by the hydrolysis of XeF4 or XeF6.
Question xviii.
Give two uses of neon and argon.
Answer:
Uses of neon (Ne) :
Uses of argon (Ar) :
Question xix.
Describe the structure of Ozone. Give two uses of ozone.
Answer:
(A)
(B) Uses of Ozone :

Question xx.
Explain the trend in following atomic properties of group 16 elements.
i. Atomic radii
ii. Ionisation enthalpy
iii. Electronegativity.
Answer:
(1) Atomic and ionic radii :
(2) Ionisation enthalpy :
Group 15 : (valence shell) ns2 npx1 npy1 npz1
Group 16 : (valence shell) ns2 npx2 npy1 npz1
Group 15 elements have extra stability of half-filled and more symmetrical orbitals, while group 16 elements acquire extra stability by losing one of paired electrons from npx- orbital forming half-filled p-orbitals.
Hence group 16 elements have lower first ionisation enthalpy than group 15 elements.
(3) Electronegativity :
4. Answer the following.
Question i.
Distinguish between rhombic sulfur and monoclinic sulfur.
Answer:

Question ii.
Give two reactions showing oxidizing property of concentrated H2SO4.
Answer:
Hot and concentrated H2SO4 acts as an oxidising agent, since it gives nascent oxygen on heating.
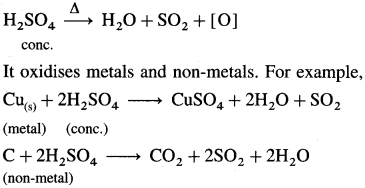
Question iii.
How is SO2 prepared in the laboratory from sodium sulfite? Give two physical properties of SO2.
Answer:
(A) Laboratory method (From sulphite) :
(B) Physical properties of SO2
Question iv.
Describe the manufacturing of H2SO4 by contact process.
Answer:
Contact process of the manufacture of sulphuric acid involves following steps :
(1) Preparation of SO2 : Sulphur or pyrite ores like iron pyrites, FeS2 on burning in excess of air, form SO2.
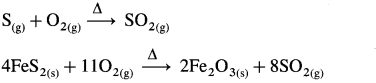
(2) Oxidation of SO2 to SO3 : SO2 is oxidised to SO3 in the presence of a heterogeneous catalyst V2O5 and atmospheric oxygen. This oxidation reaction is reversible.
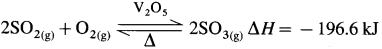
To avoid the poisoning of a costly catalyst, it is necessary to make SO2 free from the impurities like dust, moisture, As2O3 poison, etc.
The forward reaction is exothermic and favoured by increase in pressure. The reaction is carried out at high pressure (2 bar) and 720 K temperature. The reacting gases, SO2 and O2 are taken in the ratio 2:3.
(3) Dissolution of SO3 : SO3 obtained from catalytic converter is absorbed in 98%. H2SO4 to obtain H2S2O7, oleum or fuming sulphuric acid.
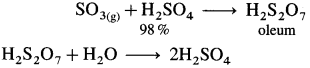
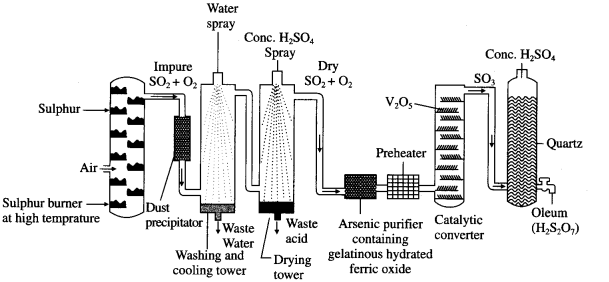
Flow diagram for the manufacture of sulphuric acid
Question 7.1 (Textbook Page No 141)
12th Chemistry Digest Chapter 7 Elements of Groups 16, 17 and 18 Intext Questions and Answers
Question 1.
Elements of group 16 generally show lower values of first ionisation enthalpy compared to the elements of corresponding period of group 15. Why?
Answer:
Group 15 elements have extra stable, half filled p-orbitals with electronic configuration (ns2np3). Therefore more amount of energy is required to remove an electron compared to that of the partially filled orbitals (ns2np4) of group 16 elements of the corresponding period.

Question 7.2 (Textbook Page No 141)
Question 1.
The values of first ionisation enthalpy of S and Cl are 1000 and 1256 kJ mol-1, respectively. Explain the observed trend.
Answer :
The elements S and Cl belong to second period of the periodic table.
Across a period effective nuclear charge increases and atomic size decreases with increase in atomic number. Therefore the energy required for the removal of electron from the valence shell (I.E.) increases in the order S < Cl.
Question 7.4 (Textbook Page No 141)
Question 1.
Fluorine has less negative electron gain affinity than chlorine. Why?
Answer :
The size of fluorine atom is smaller than chlorine atom. As a result, there are strong inter electronic repulsions in the relatively small 2p orbitals of fluorine and therefore, the incoming electron does not experience much attraction. Thus fluorine has less negative electron gain affinity than chlorine.
Try this… (Textbook Page No 140)
Question 1.
Explain the trend in the following properties of group 17 elements.
(1) Atomic size,
(2) Ionisation enthalpy,
(3) Electronegativity,
(4) Electron gain enthalpy.
Answer:
(1) Atomic size :
(2) Ionisation enthalpy :
(3) Electronegativity :
(4) Electron gain enthalpy (ΔegH) :

Question 2.
Oxygen has less negative electron gain enthalpy than sulphur. Why?
Answer:
Question 7.3 (Textbook Page No 141)
Question 1.
Why is there a large difference between the melting and boiling points of oxygen and sulphur?
Answer :
Oxygen exists as diatomic molecule (O2) whereas sulphur exists as polyatomic molecule (S8). The van der Waals forces of attraction between O2 molecules are relatively weak owing to their much smaller size. The large van der Waals attractive forces in the S8 molecules are due to large molecular size. Therefore oxygen has low m.p. and b.p. as compared to sulphur.
Question 7.5 (Textbook Page No 141)
Question 1.
Bond dissoClation enthalpy of F2 (158.8 kj mol-1) is lower than that of Cl2 (242.6 kj mol-1) Why?
Answer :
Fluorine has small atomic size than chlorine. The lone pairs on each F atom in F2 molecule are so close together that they strongly repel each other, and make the F – F bond weak. Thus, it requires less amount of energy to break the F – F bond. In Cl2 molecule the lone pairs on each Cl atom are at a larger distance and the repulsion is less.
Thus Cl – Cl bond is comparatively stronger. Therefore bond dissoClation enthalpy of F2 is lower than that of Cl2.
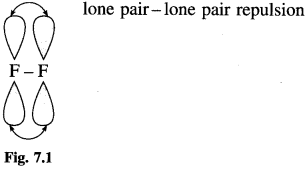
Question 7.6 (Textbook Page No 142)
Question 1.
Noble gases have very low melting and boiling points. Why?
Answer :
Noble gases are monoatomic, the only type of interatomic interactions which exist between them are weak van der Waals forces. Therefore, they can be liquefied at very low temperatures and have very low melting or boiling points.
Can you tell? (Textbook Page No 142)
Question 1.
The first member of the a group usually differs in properties from the rest of the members of the group. Why?
Answer:
The first member of a group usually differs in properties from the rest of the members of the group for the following reasons :
Use your brain power! (Textbook Page No 142)
Question 1.
Oxygen forms only OF2 with fluorine while sulphur forms SF6. Explain. Why?
Answer:
Question 2.
Which of the following possesses hydrogen bonding? H2S, H2O, H2Se, H2Te
Answer:

Question 3.
Show hydrogen bonding in the above molecule with the help of a diagram.
Answer:
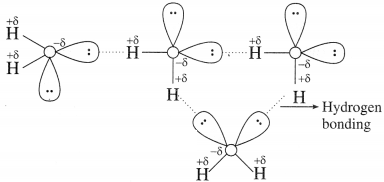
Try this….. (Textbook Page No 143)
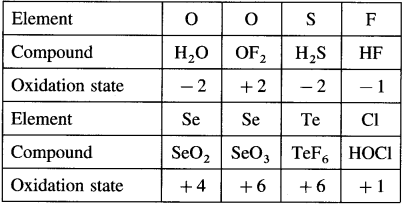
Question 1.
Complete the following tables :
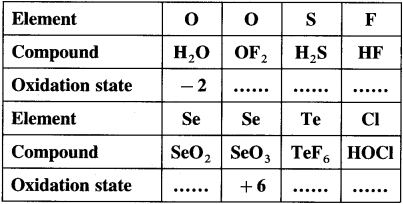
Answer:
Can you tell? (Textbook Page No 146)
Question 1.
What is allotropy?
Answer:
The property of some elements to exist in two or more different forms in the same physical state is called allotropy.
Question 2.
What is the difference between allotropy and polymorphism?
Answer:
Question 7.7 (Textbook Page No 146)
Which form of sulphur shows paramagnetic behaviour?
Answer :
In the vapour state, sulphur partly exists as S2 molecule, which has two unpaired electrons in the antibonding π* orbitals like O2. Hence it exhibits paramagnetism.
Try this….. (Textbook Page No 149)
Question 1.
Why water in a fish pot needs to be changed from time to time?
Answer:
A fish pot is an artificial ecosystem and the fish in it are selective and maintained in a restricted environment.
In a fish pot, the unwanted food and waste generated by the fish mix with the water and remain untreated due to lack of decomposers.
Accumulation of waste material will decrease the levels of dissolved oxygen in the water pot.
Hence, it is necessary to change the water from time to time.

Question 7.8 (Textbook Page No 149)
Dioxygen is paramagnetic in spite of having an even number of electrons. Explain.
Answer :
Dioxygen is a covalently bonded molecule.
The paramagnetic behaviour of O2 can be explained with the help of molecular orbital theory.
Electronic configuration O2
KK σ(2s)2 σ(2s)2 σ*(2pz)2 π(2px)2 π(2px)2 π(2py)2 π*(2px)1 π*(2py)1. Presence of two unpaired electrons in antibonding orbitals explains paramagnetic nature of dioxygen.
Question 7.9 (Textbook Page No 150)
High concentration of ozone can be dangerously explosive. Explain.
Answer :
Thermal stability : Ozone is thermodynamically unstable than oxygen and decomposes into O2. The decomposition is exothermic and results in the liberation of heat (ΔH is – ve) and an increase in entropy (ΔS is positive). This results in large negative Gibbs energy change (ΔG). Therefore high concentration of ozone can be dangerously explosive. Eq O3 → O2 + O
Try this…… (Textbook Page No 151)
(a) Ozone is used as a bleaching agent. Explain.
Answer:
(b) Why does ozone act as a powerful oxidising agent?
Answer:
Ozone decomposes to liberate nascent oxygen, hence it is a powerful oxidising agent. O3(g) → O2(g) + O
For example :
Question 7.10 : (Textbook Page No 154)
What is the action of concentrated H2SO4 on (a) HBr (b) HI
Answer :
Concentrated sulphuric acid oxidises hydrobromic acid to bromine.
2HBr + H2SO4 → Br2 + SO2 + 2H2O
It oxidises hydroiodic acid to iodine.
2HI + H2SO4 → I2 + SO2 + 2H2O
Try this….. (Textbook Page No 156)
Question 1.
Give the reasons for the bleaching action of chlorine.
Answer:
Question 2.
Name two gases used in war.
Answer:
Phosgene : COCl2
Mustard gas: Cl – CH2 – CH2 – S – CH2 – CH2 – Cl

Use your brain power! (Textbook Page No 157)
Question 1.
Chlorine and fluorine combine to form interhalogen compounds. The halide ion will be of chlorine or fluorine?
Answer:
Among the- two halogens, chlorine is more electropositive than fluorine (Electronegativity values: F = 4.0, Cl = 3.2)
The interhalogen compound is regarded as the halide of the more electropositive halogen. Hence, the interhalogen compound is the fluoride of chlorine, i.e. chlorine monofluoride, CiF.
Question 2.
Why does fluorine combine with other halogens to form maximum number of fluorides?
Answer:
Since fluorine is the most electronegative element and has the smallest atomic radius compared to other halogen fluorine forms maximum number of fluorides.
Use your brain power! (Textbook Page No 158)
Question 1.
What will be the names of the following compounds: ICl, BrF?
Answer:
ICl : Iodine monochloride
BrF : Bromine monofluoride
Question 2.
Which halogen (X) will have maximum number of other halogen (X ) attached?
Answer:
The halogen Iodine (I) will have the maximum number of other halogens attached.
Question 3.
Which halogen has tendency to form more interhalogen compounds?
Answer:
The halogen fluorine (F) has the maximum tendency to form more interhalogen compounds as it has a small size and more electronegativity.
Question 4.
Which will be more reactive?
(a) ClF3 or ClF,
(b) BrF5 or BrF
Answer:
ClF3 is more reactive than ClF, while BrF5 is more reactive than BrF. Both ClF3 and BrF5 are unstable compared to ClF and BrF respectively due to steric hindrance hence are more reactive.
Question 5.
Complete the table :
Answer:

Use your brain power! (Textbook Page No 159)
Question 1.
In the special reaction for ICl, identify the oxidant and the reductant? Denote oxidation states of the species.
Answer:
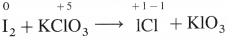
Potassium chlorate, KClO3 is the oxidising agent or oxidant and iodine is the reducing agent or reductant.
Use your brain power! (Textbook Page No 162)
Question 1.
What are missing entries?
Answer:
Chapter 7 Elements of Groups 16, 17 and 18 Textbook Exercise Questions and Answers.
1. Select appropriate answers for the following.
Question i.
Which of the following has the highest electron gain enthalpy?
A. Fluorine
B. Chlorine
C. Bromine
D. Iodine
Answer:
B. Chlorine

Question ii.
Hydrides of group 16 are weakly acidic. The correct order of acidity is
A. H2O > H2S > H2Se > H2Te
B. H2Te > H2O > H2S > H2Se
C. H2Te > H2Se > H2S > H2O
D. H2Te > H2Se > H2O > H2S
Answer:
C. H2Te > H2Se > H2S > H2O
Question iii.
Which of the following element does not show oxidation state of +4 ?
A. O
B. S
C. Se
D. Te
Answer:
A. O
Question iv.
HI acid when heated with conc. H2SO4 forms
A. HIO3
B. KIO3
C. I2
D. KI
Answer:
C. I2
Question v.
Ozone layer is depleted by
A. NO
B. NO2
C. NO3
D. N2O5
Answer:
A. NO
Question vi.
Which of the following occurs in liquid state at room temperature?
A. HIO3
B. HBr
C. HCl
D. HF
Answer:
D. HF
Question vii.
In pyrosulfurous acid oxidation state of sulfur is
A. Only +2
B. Only +4
C. +2 and +6
D. Only +6
Answer:
B. Only + 4
Question viii.
Stability of interhalogen compounds follows the order
A. BrF > IBr > ICl > ClF > BrCl
B. IBr > BeF > ICl > ClF > BrCl
C. ClF > ICl > IBr > BrCl > BrF
D. ICl > ClF > BrCl > IBr > BrF
Answer:
C. ClF > ICl > IBr > BrCl > BrF
Question ix.
BrCl reacts with water to form
A. HBr
B. Br2 + Cl2
C. HOBr
D. HOBr + HCl
Answer:
D. HOBr + HCl

Question x.
Chlorine reacts with excess of fluorine to form.
A. ClF
B. ClF3
C. ClF2
D. Cl2F3
Answer:
B. ClF3
Question xi.
In interhalogen compounds, which of the following halogens is never the central atom.
A. I
B. Cl
C. Br
D. F
Answer:
D. F
Question xii.
Which of the following has one lone pair of electrons?
A. IF3
B. ICl
C. IF5
D. ClF3
Answer:
C. IF5
Question xiii.
In which of the following pairs, molecules are paired with their correct shapes?
A. [I3] : bent
B. BrF5 : trigonal bipyramid
C. ClF3 : trigonal planar
D. [BrF4] : square planar
Answer:
A. [I3] : bent
Question xiv.
Among the known interhalogen compounds, the maximum number of atoms is
A. 3
B. 6
C. 7
D. 8
Answer:
D. 8
2. Answer the following.
Question i.
Write the order of the thermal stability of the hydrides of group 16 elements.
Answer:
The thermal stability of the hydrides of group 16 elements decreases in the order of H2O > H2S > H2Se > H2Te.
Question ii.
What is the oxidation state of Te in TeO2?
Answer:
The oxidation state of Te in TeO2 is + 4.
Question iii.
Name two gases which deplete ozone layer.
Answer:
Nitrogen oxide (NO) released from exhaust systems of car or supersonic jet aeroplanes and chlorofluorocarbons (Freons) used in aerosol sprays and refrigerators deplete ozone layer.
Question iv.
Give two uses of ClO2
Answer:
(i) ClO2 is used as a bleaching agent for paper pulp and textiles.
(ii) It is also used in water treatment.
Question v.
What is the action of bromine on magnesium metal?
Answer:
Bromine reacts instantly with magnesium metal to give magnesium bromide.


Question vi.
Write the names of allotropic forms of selenium.
Answer:
Selenium has two allotropic forms as follows :
(i) Red (non-metallic) form
(ii) Grey (metallic) form
Question vii.
What is the oxidation state of S in H2SO4.
Answer:
The oxidation state of S in H2SO4 is + 6.
Question viii.
The pKa values of HCl is -7.0 and that of HI is -10.0. Which is the stronger acid?
Answer:
For HCl, pKa = -7.0, hence its dissoClation constant is, Ka = 1 x 10-7.
For HI pKa = – 10.0, hence its dissoClation constant is Ka = 1 x 10-7. Hence HCl dissoClates more than HI.
Therefore HCl is a stronger acid than HI.
Question ix.
Give one example showing reducing property of ozone.
Answer:
Ozone decomposes to liberate nascent oxygen, hence it is a powerful oxidising agent. O3(g) → O2(g) + O
For example :
(i) It oxidises lead sulphide (PbS) to lead sulphate (PbSO4).
pbS(s) + 4O3(g) → PbSO(s) + 4O2(g)
(ii) Potassium iodide, KI is oxidised to iodine, I2 in the solution.
2KI(aq) + H2O(1) + O3(g) → 2KOH(aq) + I2(s) + O2(g)
Question x.
Write the reaction of conc. H2SO4 with sugar.
Answer:
Concentrated sulphuric acid when added to sugar, it is dehydrated giving carbon.
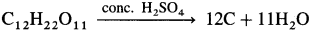
The carbon that is left behind is called sugar charcoal and the process is called char.
Question xi.
Give two uses of chlorine.
Answer:
Chlorine is used for :
Question xii.
Complete the following.
1. ICl3 + H2O …….. + …….. + ICl
2. I2 + KClO3 ……. + KIO2
3. BrCl + H2O ……. + HCl
4. Cl2 + ClF3 ……..
5. H2C = CH2 + ICl …….
6. XeF4 + SiO2 ……. + SiF4
7. XeF6 + 6H2O …….. + HF
8. XeOF4 + H2O ……. + HF
Answer:
1. 2ICI3 + 3H2O → 5HCl + HlO3 + ICl
2. I2 + KCIO3 → ICl + KIO3
3. BrCl + H2O → HOBr + HCl
4. Cl2 + C1F3 → 3ClF
5. CH2 = CH2 + ICl → CH2I – CH2Cl
6. 2XeF6 + SiO2 → 2XeOF4 + SiF4
7. XeF6 + 3H2O → XeO3 + 6HF
8. XeOF4 + H2O→ XeO2F2 + 2HF

Question xiii.
Match the following
A – B
XeOF2 – Xenon trioxydifluoride
XeO2F2 – Xenon monooxydifluoride
XeO3F2 – Xenon dioxytetrafluoride
XeO2F4 – Xenon dioxydifluoride
Answer:
XeOF2 – Xenon monooxydifluoride
XeO2F2 – Xenon dioxydifluoride
XeO3F2 – Xenon trioxydifluoride
XeO2F4 – Xenon dioxytetrafluoride
Question xiv.
What is the oxidation state of xenon in the following compounds?
XeOF4, XeO3, XeF5, XeF4, XeF2.
Answer:
| Compound | Oxidation state of Xe |
| XeOF4 | + 6 |
| XeO3 | + 6 |
| XeF6 | + 6 |
| XeF4 | + 4 |
| XeF2 | + 2 |
Question i.
The first ionisation enthalpies of S, Cl and Ar are 1000, 1256 and 1520 kJ/mol-1, respectively. Explain the observed trend.
Answer:
(i) The atomic number increases as, 16S < 17Cl < 18Ar1.
(ii) Due to decrease in atomic size and increase in effective nuclear charge, Cl binds valence electrons strongly.
(iii) Hence ionisation enthalpy of Cl (1256 kJ mol-1) is higher than that of S(1000 kJ mol-1)
(iv) Ar has electronic configuration 3s23p6. Since all electrons are paired and the octet is complete, it has the highest ionisation enthalpy, (1520 kJ mol-1)
Question ii.
“Acidic character of hydrides of group 16 elements increases from H2O to H2Te” Explain.
Answer:
(i) The thermal stability of the hydrides of group 16 elements decreases from H2O to H2Te. This is because the bond dissociation enthalpy of the H-E bond decreases down the group.
(ii) Thus, the acidic character increases from H2O to H2Te.
Question iii.
How is dioxygen prepared in laboratory from KClO3?
Answer:
By heating chlorates, nitrates and permanganates.
Potassium chlorate in the presence of manganese dioxide on heating decomposes to form potassium chloride and oxygen.
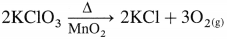
Question iv.
What happens when
a. Lead sulfide reacts with ozone (O3).
b. Nitric oxide reacts with ozone.
Answer:
(i) It oxidises lead sulphide (PbS) to lead sulphate (PbSO4) changing the oxidation state of S from – 2 to +6.
PbS(s) + 4O3(g) → PbSO(s) + 4O2(g)
(ii) Ozone oxidises nitrogen oxide to nitrogen dioxide.
NO(g) + O3(g) → NO2(g) + O2(g)
Question v.
Give two chemical reactions to explain oxidizing property of concentrated H2SO4.
Answer:
Hot and concentrated H2SO4 acts as an oxidising agent, since it gives nascent oxygen on heating.
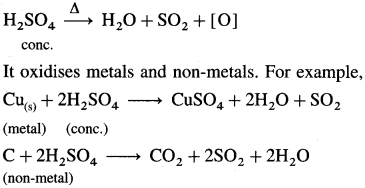

Question vi.
Discuss the structure of sulfur dioxide.
Answer:
(i) SO2 molecule has a bent V shaped structure with S-O-S bond angle 119.5° and bond dissoClation enthalpy is 297 kJ mol-1.
(ii) Sulphur in SO2 is sp2 hybridised forming three hybrid orbitals. Due to lone pair electrons, bond angle is reduced from 120° to 119.5°.
(iii) In SO2, each oxygen atom is bonded to sulphur by σ and a π bond.
(iv) a bond between S and O are formed by sp2-p overlapping.
(v) One of π bonds is formed by pπ – pπ overlapping while other n bond is formed by pπ – dπ overlap.
(vi) Due to resonance both the bonds are identical having observed bond length 143 pm due to resonance,

Question vii.
Fluorine shows only -1 oxidation state while other halogens show -1, +1, +3, +5 and +7 oxidation states. Explain.
Answer:
Question viii.
What is the action of chlorine on the following
a. Fe
b. Excess of NH3
Answer:
(a) Chlorine reacts with Fe to give ferric chloride.
2Fe + 3Cl2 → 2FeCl3
(b) Chlorine reacts with the excess of ammonia to form ammonium chloride, NH4Cl and nitrogen.

Question ix.
How is hydrogen chloride prepared from sodium chloride?
Answer:

Question x.
Draw structures of XeF6, XeO3, XeOF4, XeF2.
Answer:


Question xi.
What are interhalogen compounds? Give two examples.
Answer:
Interhalogen compounds : Compounds formed by the combination of atoms of two different halogens are called interhalogen compounds. In an interhalogen compound, of the two halogen atoms, one atom is more electropositive than the other. The interhalogen compound is regarded as the halide of the more electropositive halogen.
For example ClF, BrF3, ICl
Question xii.
What is the action of hydrochloric acid on the following?
a. NH3
b. Na2CO3
Answer:
a. Hydrochloric acid reacts with ammonia to give white fumes of ammonium chloride.
NH3 + HCl → NH4Cl
b. Hydrochloric acid reacts with sodium carbonate to give sodium chloride, water with the liberation of carbon dioxide gas.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Question xiii.
Give two uses of HCl.
Answer:
Hydrogen chloride (OR hydrochloric acid) is used :
Question xiv.
Write the names and structural formulae of oxoacids of chlorine.
Answer:



Question xv.
What happens when
a. Cl2 reacts with F2 in equal volume at 437 K.
b. Br2 reacts with excess of F2.
Answer:
(a) Cl2 reacts with F2 in equal volumes at 437 K to give chlorine monofluoride ClF.
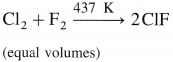
(b) Br2 reacts with excess of F2 to give bromine trifluoride BF3.
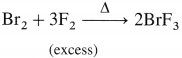
Question xvi.
How are xenon fluorides XeF2, XeF4 and XeF6 obtained ? Give suitable reactions.
Answer:
Xenon fluorides are generally prepared by the direct reaction of xenon and fluorine in different ratios and under appropriate experimental conditions, such as temperature, in the presence of an electric discharge and by a photochemical reaction.
(i) Preparation of XeF2 :
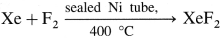
(ii) Preparation of XeF4 :

(iii) Preparation of XeF6 :

Question xvii.
How are XeO3 and XeOF4 prepared?
Answer:
Preparation of XeO3 : Xenon trioxide (XeO3) is prepared by the hydrolysis of XeF4 or XeF6.
Question xviii.
Give two uses of neon and argon.
Answer:
Uses of neon (Ne) :
Uses of argon (Ar) :
Question xix.
Describe the structure of Ozone. Give two uses of ozone.
Answer:
(A)
(B) Uses of Ozone :

Question xx.
Explain the trend in following atomic properties of group 16 elements.
i. Atomic radii
ii. Ionisation enthalpy
iii. Electronegativity.
Answer:
(1) Atomic and ionic radii :
(2) Ionisation enthalpy :
Group 15 : (valence shell) ns2 npx1 npy1 npz1
Group 16 : (valence shell) ns2 npx2 npy1 npz1
Group 15 elements have extra stability of half-filled and more symmetrical orbitals, while group 16 elements acquire extra stability by losing one of paired electrons from npx- orbital forming half-filled p-orbitals.
Hence group 16 elements have lower first ionisation enthalpy than group 15 elements.
(3) Electronegativity :
| Elements | O | S | Se | Te | Po |
| Electronegativity | 3.5 | 2.44 | 2.48 | 2.01 | 1.76 |
Question i.
Distinguish between rhombic sulfur and monoclinic sulfur.
Answer:
| Rhombic sulphur | Monoclinic sulphur |
| 1. It is pale yellow. | 1. It is bright yellow. |
| 2. Orthorhombic crystals | 2. Needle-shaped monoclinic crystals |
| 3. Melting point, 385.8 K | 3. Melting point, 393 K |
| 4. Density, 2.069 g/cm3 | 4. Density: 1.989 g/cm3 |
| 5. Insoluble in water, but soluble in CS2 | 5. Soluble in CS2 |
| 6. It is stable below 369 K and transforms to α-sulphur above this temperature. | 6. It is stable above 369 K and transforms into β-sulphur below this temperature. |
| 7. It exists as S8 molecules with a structure of a puckered ring. | 7. It exists as S8 molecules with a structure of a puckered ring. |
| 8. It is obtained by the evaporation of roll sulphur in CS2 | 8. It is prepared by melting rhombic sulphur and cooling it till a crust is formed. Two holes are pierced in the crust and the remaining liquid is poured to obtain needle-shaped crystals of monoclinic sulphur (β-sulphur). |

Question ii.
Give two reactions showing oxidizing property of concentrated H2SO4.
Answer:
Hot and concentrated H2SO4 acts as an oxidising agent, since it gives nascent oxygen on heating.
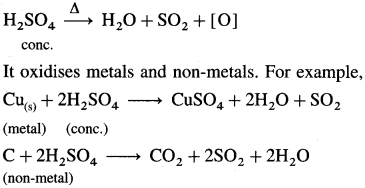
Question iii.
How is SO2 prepared in the laboratory from sodium sulfite? Give two physical properties of SO2.
Answer:
(A) Laboratory method (From sulphite) :
(B) Physical properties of SO2
Question iv.
Describe the manufacturing of H2SO4 by contact process.
Answer:
Contact process of the manufacture of sulphuric acid involves following steps :
(1) Preparation of SO2 : Sulphur or pyrite ores like iron pyrites, FeS2 on burning in excess of air, form SO2.
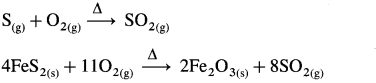
(2) Oxidation of SO2 to SO3 : SO2 is oxidised to SO3 in the presence of a heterogeneous catalyst V2O5 and atmospheric oxygen. This oxidation reaction is reversible.
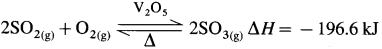
To avoid the poisoning of a costly catalyst, it is necessary to make SO2 free from the impurities like dust, moisture, As2O3 poison, etc.
The forward reaction is exothermic and favoured by increase in pressure. The reaction is carried out at high pressure (2 bar) and 720 K temperature. The reacting gases, SO2 and O2 are taken in the ratio 2:3.
(3) Dissolution of SO3 : SO3 obtained from catalytic converter is absorbed in 98%. H2SO4 to obtain H2S2O7, oleum or fuming sulphuric acid.
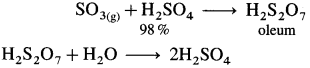

Flow diagram for the manufacture of sulphuric acid
Question 7.1 (Textbook Page No 141)
12th Chemistry Digest Chapter 7 Elements of Groups 16, 17 and 18 Intext Questions and Answers
Question 1.
Elements of group 16 generally show lower values of first ionisation enthalpy compared to the elements of corresponding period of group 15. Why?
Answer:
Group 15 elements have extra stable, half filled p-orbitals with electronic configuration (ns2np3). Therefore more amount of energy is required to remove an electron compared to that of the partially filled orbitals (ns2np4) of group 16 elements of the corresponding period.

Question 7.2 (Textbook Page No 141)
Question 1.
The values of first ionisation enthalpy of S and Cl are 1000 and 1256 kJ mol-1, respectively. Explain the observed trend.
Answer :
The elements S and Cl belong to second period of the periodic table.
Across a period effective nuclear charge increases and atomic size decreases with increase in atomic number. Therefore the energy required for the removal of electron from the valence shell (I.E.) increases in the order S < Cl.
Question 7.4 (Textbook Page No 141)
Question 1.
Fluorine has less negative electron gain affinity than chlorine. Why?
Answer :
The size of fluorine atom is smaller than chlorine atom. As a result, there are strong inter electronic repulsions in the relatively small 2p orbitals of fluorine and therefore, the incoming electron does not experience much attraction. Thus fluorine has less negative electron gain affinity than chlorine.
Try this… (Textbook Page No 140)
Question 1.
Explain the trend in the following properties of group 17 elements.
(1) Atomic size,
(2) Ionisation enthalpy,
(3) Electronegativity,
(4) Electron gain enthalpy.
Answer:
(1) Atomic size :
(2) Ionisation enthalpy :
| Element | F | Cl | Br | I |
| Ionisation enthalpy kJ/mol | 1680 | 1256 | 1142 | 1008 |
(4) Electron gain enthalpy (ΔegH) :

Question 2.
Oxygen has less negative electron gain enthalpy than sulphur. Why?
Answer:
Question 7.3 (Textbook Page No 141)
Question 1.
Why is there a large difference between the melting and boiling points of oxygen and sulphur?
Answer :
Oxygen exists as diatomic molecule (O2) whereas sulphur exists as polyatomic molecule (S8). The van der Waals forces of attraction between O2 molecules are relatively weak owing to their much smaller size. The large van der Waals attractive forces in the S8 molecules are due to large molecular size. Therefore oxygen has low m.p. and b.p. as compared to sulphur.
Question 7.5 (Textbook Page No 141)
Question 1.
Bond dissoClation enthalpy of F2 (158.8 kj mol-1) is lower than that of Cl2 (242.6 kj mol-1) Why?
Answer :
Fluorine has small atomic size than chlorine. The lone pairs on each F atom in F2 molecule are so close together that they strongly repel each other, and make the F – F bond weak. Thus, it requires less amount of energy to break the F – F bond. In Cl2 molecule the lone pairs on each Cl atom are at a larger distance and the repulsion is less.
Thus Cl – Cl bond is comparatively stronger. Therefore bond dissoClation enthalpy of F2 is lower than that of Cl2.

Question 7.6 (Textbook Page No 142)
Question 1.
Noble gases have very low melting and boiling points. Why?
Answer :
Noble gases are monoatomic, the only type of interatomic interactions which exist between them are weak van der Waals forces. Therefore, they can be liquefied at very low temperatures and have very low melting or boiling points.
Can you tell? (Textbook Page No 142)
Question 1.
The first member of the a group usually differs in properties from the rest of the members of the group. Why?
Answer:
The first member of a group usually differs in properties from the rest of the members of the group for the following reasons :
Use your brain power! (Textbook Page No 142)
Question 1.
Oxygen forms only OF2 with fluorine while sulphur forms SF6. Explain. Why?
Answer:
Question 2.
Which of the following possesses hydrogen bonding? H2S, H2O, H2Se, H2Te
Answer:

Question 3.
Show hydrogen bonding in the above molecule with the help of a diagram.
Answer:

Try this….. (Textbook Page No 143)
Question 1.
Complete the following tables :

Answer:

Can you tell? (Textbook Page No 146)
Question 1.
What is allotropy?
Answer:
The property of some elements to exist in two or more different forms in the same physical state is called allotropy.
Question 2.
What is the difference between allotropy and polymorphism?
Answer:
Question 7.7 (Textbook Page No 146)
Which form of sulphur shows paramagnetic behaviour?
Answer :
In the vapour state, sulphur partly exists as S2 molecule, which has two unpaired electrons in the antibonding π* orbitals like O2. Hence it exhibits paramagnetism.
Try this….. (Textbook Page No 149)
Question 1.
Why water in a fish pot needs to be changed from time to time?
Answer:
A fish pot is an artificial ecosystem and the fish in it are selective and maintained in a restricted environment.
In a fish pot, the unwanted food and waste generated by the fish mix with the water and remain untreated due to lack of decomposers.
Accumulation of waste material will decrease the levels of dissolved oxygen in the water pot.
Hence, it is necessary to change the water from time to time.

Question 7.8 (Textbook Page No 149)
Dioxygen is paramagnetic in spite of having an even number of electrons. Explain.
Answer :
Dioxygen is a covalently bonded molecule.
The paramagnetic behaviour of O2 can be explained with the help of molecular orbital theory.
Electronic configuration O2
KK σ(2s)2 σ(2s)2 σ*(2pz)2 π(2px)2 π(2px)2 π(2py)2 π*(2px)1 π*(2py)1. Presence of two unpaired electrons in antibonding orbitals explains paramagnetic nature of dioxygen.
Question 7.9 (Textbook Page No 150)
High concentration of ozone can be dangerously explosive. Explain.
Answer :
Thermal stability : Ozone is thermodynamically unstable than oxygen and decomposes into O2. The decomposition is exothermic and results in the liberation of heat (ΔH is – ve) and an increase in entropy (ΔS is positive). This results in large negative Gibbs energy change (ΔG). Therefore high concentration of ozone can be dangerously explosive. Eq O3 → O2 + O
Try this…… (Textbook Page No 151)
(a) Ozone is used as a bleaching agent. Explain.
Answer:
(b) Why does ozone act as a powerful oxidising agent?
Answer:
Ozone decomposes to liberate nascent oxygen, hence it is a powerful oxidising agent. O3(g) → O2(g) + O
For example :
Question 7.10 : (Textbook Page No 154)
What is the action of concentrated H2SO4 on (a) HBr (b) HI
Answer :
Concentrated sulphuric acid oxidises hydrobromic acid to bromine.
2HBr + H2SO4 → Br2 + SO2 + 2H2O
It oxidises hydroiodic acid to iodine.
2HI + H2SO4 → I2 + SO2 + 2H2O
Try this….. (Textbook Page No 156)
Question 1.
Give the reasons for the bleaching action of chlorine.
Answer:
Question 2.
Name two gases used in war.
Answer:
Phosgene : COCl2
Mustard gas: Cl – CH2 – CH2 – S – CH2 – CH2 – Cl

Use your brain power! (Textbook Page No 157)
Question 1.
Chlorine and fluorine combine to form interhalogen compounds. The halide ion will be of chlorine or fluorine?
Answer:
Among the- two halogens, chlorine is more electropositive than fluorine (Electronegativity values: F = 4.0, Cl = 3.2)
The interhalogen compound is regarded as the halide of the more electropositive halogen. Hence, the interhalogen compound is the fluoride of chlorine, i.e. chlorine monofluoride, CiF.
Question 2.
Why does fluorine combine with other halogens to form maximum number of fluorides?
Answer:
Since fluorine is the most electronegative element and has the smallest atomic radius compared to other halogen fluorine forms maximum number of fluorides.
Use your brain power! (Textbook Page No 158)
Question 1.
What will be the names of the following compounds: ICl, BrF?
Answer:
ICl : Iodine monochloride
BrF : Bromine monofluoride
Question 2.
Which halogen (X) will have maximum number of other halogen (X ) attached?
Answer:
The halogen Iodine (I) will have the maximum number of other halogens attached.
Question 3.
Which halogen has tendency to form more interhalogen compounds?
Answer:
The halogen fluorine (F) has the maximum tendency to form more interhalogen compounds as it has a small size and more electronegativity.
Question 4.
Which will be more reactive?
(a) ClF3 or ClF,
(b) BrF5 or BrF
Answer:
ClF3 is more reactive than ClF, while BrF5 is more reactive than BrF. Both ClF3 and BrF5 are unstable compared to ClF and BrF respectively due to steric hindrance hence are more reactive.
Question 5.
Complete the table :
| Formula | Name |
| ClF | Chlorine monofluoride |
| ClF3 | ………………………………… |
| ………………………………… | Chlorine pentachloride |
| BrF | ………………………………… |
| ………………………………… | Bromine pentafluoride |
| ICl | ………………………………… |
| ICl3 | ………………………………… |
| Formula | Name |
| ClF | Chlorine monofluoride |
| ClF3 | Chlorine trifluoride |
| CIF5 | Chlorine pentafluoride |
| BrF | Bromine monofluoride |
| BrF5 | Bromine pentafluoride |
| ICl | Iodine monochloride |
| ICl3 | Iodine trichloride |

Use your brain power! (Textbook Page No 159)
Question 1.
In the special reaction for ICl, identify the oxidant and the reductant? Denote oxidation states of the species.
Answer:

Potassium chlorate, KClO3 is the oxidising agent or oxidant and iodine is the reducing agent or reductant.
Use your brain power! (Textbook Page No 162)
Question 1.
What are missing entries?
| Formula | Name |
| XeOF2 …………… XeO3F2 XeO2F4 |
Xenon monooxyfluoride Xenon dioxydifluoride …………………………………….. …………………………………….. |
| Formula | Name |
| XeOF2 XeO2F2 XeO3F2 XeO2F4 |
Xenon monooxydifluoride Xenon dioxydifluoride Xenon trioxydifluoride Xenon dioxytetrafluoride |