Balbharti Maharashtra State Board Class 7 Science Solutions Chapter 14 Elements, Compounds and Mixtures Notes, Textbook Exercise Important Questions and Answers.
Std 7 Science Chapter 14 Elements, Compounds and Mixtures Question Answer Maharashtra Board
Class 7 Science Chapter 14 Elements, Compounds and Mixtures Question Answer Maharashtra Board
1. Who are my companions?
Question a.
| Column ‘A’ | Column B’ |
| 1. Stainless steel | a. Non-metal |
| 2. Silver | b. Compound |
| 3. Bhajani mixture for milling | c. Mixture |
| 4. Salt | d. Element |
| 5. Coal | e. Alloy |
| 6. Hydrogen | f. Metal |
Answer:
| Column ‘A’ | Column B’ |
| 1. Stainless steel | e. Alloy |
| 2. Silver | f. Metal |
| 3. Bhajani mixture for milling | c. Mixture |
| 4. Salt | b. Compound |
| 5. Coal | a. Non-metal |
| 6. Hydrogen | d. Element |

2. Write the names of elements from the following symbols:
Zn, Cd, Xe, Br, Ti, Cu, Fe, Si, Ir, Pt.
Question a.
Write the names of elements from the following symbols:
Zn, Cd, Xe, Br, Ti, Cu, Fe, Si, Ir, Pt.
Answer:
| Symbol | Element |
| Zn | Zinc |
| Cd | Cadmium |
| Xe | Xenon |
| Br | Bromine |
| Ti | Titanium |
| Cu | Copper |
| Fe | Iron |
| Si | Silicon |
| Ir | Iridium |
| Pt | Platinum |

3. What are the molecular formulae of the following compounds?
(Hydrochloric acid, Sulphuric acid, Sodium chloride, Glucose, Methane)
Question a.
What are the molecular formulae of the following compounds?
(Hydrochloric acid, Sulphuric acid, Sodium chloride, Glucose, Methane)
Answer:
- Hydrochloric acid → HCl
- Sulphuric acid → H 2 SO 4
- Sodium Chloride → NaCl
- Glucose → C 6 H 12 O 6
- Methane → CH 4
- Water → H 2 O
- Carbon dioxide → CO 2
- Sucrose (sugar) → C 12 H 22 O 11

4. Give scientific reasons:
Question a.
Buttermilk is churned to get butter.
Answer:
- Churning is the process of shaking up buttermilk to make butter.
- Butter is essentially the fat of milk.
- Churning physically agitates the cream until it ruptures the membrane surrounding milk fat
- Fat droplets can join with each other to form clumps of fat.
Question b.
In Chromatography the ingredients of a mixture rise up to a limited height when water rises up to the upper end of the paper.
Answer:
In Chromatography two properties of substances are used, (a) They are the stability of the substances in the solvent that moves up. The ability of the substance to stick to the stationary filter paper, (b) So all the components of the mixture do not rise all the way to the upper end of the filter paper but remain behind at limited heights.
Question c.
A wet cloth is wrapped around a water storage container in summer.
Answer:
A wet cloth will absorb the heat from the surroundings and it will keep the water in the container, cool for a longer time.

5. Explain the difference.
Question a.
Metals and Non-metals
Answer:
| Metals | Non-metals |
| 1. Metals are good conductors of heat and electricity. | 1. Non-metals are poor conductors of heat and electricity. |
| 2. Metals are solids at room temperature except for mercury. | 2. Non-metals exists in all three states. |
| 3. Metals are lustrous, (shiny) | 3. Non-metals are not lustrous, (dull appearance) except graphite. |
| 4. Metals are malleable (can be hammered into sheets) | 4. Non-metals are brittle, not malleable. |
| 5. Metals are ductile, can be drawn into wire. | 5. Non-metals are not ductile. |
| 6. Metals are very hard and strong. | 6. Non-metals are brittle, will break down into pieces except diamond. |

Question b.
Mixture and Compound
Answer:
| Mixture | Compound |
| 1. The different substances are not chemically joined together to form a mixture but mixed physically. | 1. A compound is formed when two or more elements chemically combine together e.g. H 2 O → chemical formula of water. |
| 2. Each substance in the mixture retains its own properties | 2. Compound has fixed properties. |
| 3. Mixtures are impure substances. | 3. Compounds are pure substances. |
| 4. Substances from the mixture can easily be separated by physical methods. | 4. The constituents of a compound can be separated only by chemical methods. |
| 5. The constituents of a mixture are present in varying proportions. | 5. The constituents of a compound are present in fixed proportion. |

Question c.
Atoms and Molecules
Answer:
| Atoms | Molecules |
| 1. An atom is the fundamental part of matter. | 1. A molecule is a group of chemically bonded atoms. |
| 2. Atoms are basic building blocks of matter. | 2. Molecule is the smallest unit of a chemical compound |
| 3. It is foundation of molecules. | 3. It is foundation of chemical compound. |
Question d.
Separation by distillation and Separation by separating funnel
Answer:
| Separation by distillation | Separation by separating funnel |
| 1. Distillation is used for purification of impure liquids, for separating liquid and solids (Separation of mixture containing two miscible liquids) e.g. to separate salt and water from saltwater. | 1. This procedure is used for separating two liquids e.g. separating oil and water, (two immiscible liquids) |

6. Write answers to the following questions in your own words.
Question a.
How are the components of mixture separated by simple methods?
Answer:
1. Component of mixtures are separated by straining, filtering, sifting, picking, sorting, winnowing, combing with a magnet and sublimation.
2. In a mixture, the constituent substances do not lose their identity, they can be separated easily by physical methods.
a. Sedimentation: (i) It is a process of separating an insoluble solid from a liquid in which it is suspended, by allowing it to settle to the bottom of the container, e.g. Muddy water contains heavier particles like sand and soil, (ii) Leave this muddy water undisturbed for some time, (iii) The heavier soil and sand particles settle down and the clear upper water is poured out by decantation.
b. Filtration: It is used for separating insoluble solids from a liquid, e.g. mixture of chalk and water is poured through a filter paper in a funnel while the water gets
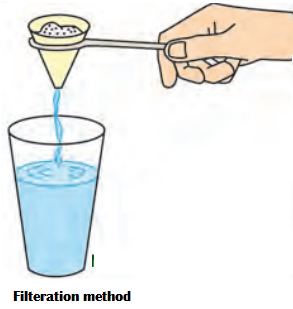
collected in the beaker below, chalk is retained in the filter paper.
c. Evaporation: It is used for recovering dissolved solid substances from solutions by evaporation e.g. sugar can be recovered from sugar-water.
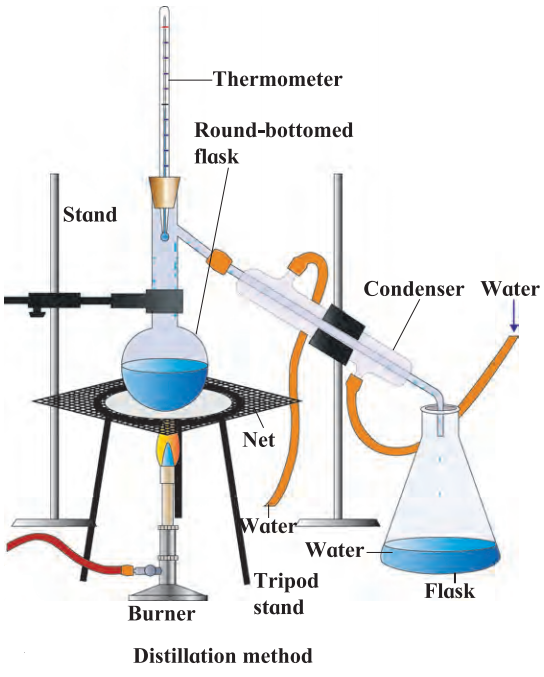
d. Distillation: It is a process of heating a solution containing soluble solids to form vapours of the liquid and then cooling the vapours to get the liquid back. e.g. A mixture of common salt and water is taken in distillation flask and heated. Steam rises up and comes out into condenser.
e. Sublimation: It is a process in which some solids on heating are transformed directly to vapour without passing through the liquid state, (i) It is used to separate a mixture of solids. The vapours are cooled separately, (ii) Used to separate ammonium chloride, iodine, camphor and sulphur from any mixture.
f. Magnetic separation: A mixture with iron fillings as one of the components can be separated using magnet to attract the iron particles away from the mixture.

Question b.
Which elements (metals, non-metals) compounds and mixtures do we use in our day to day life?
Answer:
Elements – non-metal
- Oxygen, nitrogen → present in air
- Hydrogen → present in water
- Silicon → in memory card
- Lithium → to make batteries
- Neon → in neon signs/lights
- Sulphur → used in water treatment, agricultural pesticides
Elements – metals
- Calcium → in milk
- Silver, gold → used in jewellery
- Aluminium, copper, iron → Kitchen vessels
- Mercury → in thermometer
- Copper → electric wires
Compounds:
- Sodium chloride → table salt
- Sodium carbonate → washing soda
- Sodium bicarbonate → baking soda
- Sodium hypochloride → bleaching powder
- Sodium hydroxide, Potassium hydroxide → in making soaps
- Calcium oxide, Calcium hydroxide → in white washing the buildings.
- Hydrochloric acid → in cleaning toilets
- Sucrose → sugar used in cooking and baking.
Mixtures:
- Bhel → mixture of puffed rice, sev, groundnuts, lemon juice
- Concrete → mixture of cement, sand and rocks
-
Salad → mixture of onion, cucumber, tomato, lettuce, etc.
Sherbet and saltwater are also mixtures.

Question c.
In everyday life, where and for what purpose do we use centrifugation?
Answer:
Centrifugation: It is a process which involves application of centrifugal force.
1. It is used in industrial and laboratory settings. It is used for separation of fluids, gas or liquid based on density. In centrifugation mixture is separated through spinning and the solid settles to the bottom and the solution is clear.
2. Best example of centrifugal force is working of washing machine when it dries the clothes. The wet clothes are moving in circular path and a force acts on water particles in clothes and this force pulls water all outer side. Force involved is centrifugal force which removes the water from the clothes and clothes are dried in this way.
3. Cream separation: When the machine moves, the rod set inside milk moves and milk is pressurised and solid part from this milk goes outside due to centrifugal force and this solid part is cream. In this way the cream is separated from the milk.
Question d.
Where are methods of separation by distillation and by separating funnel used?
Answer:
1. Distillation: It is the process of separating the components or substances from a compound. It is a process of heating a solution containing soluble solids to form vapours of the liquid and then cooling the vapours to get the liquid back.
Distillation method is used in the following:
- to separate components of air into oxygen, nitrogen, argon, etc.
- to separate salt from saltwater.
- to purify impure liquids.
- to prepare distilled water.
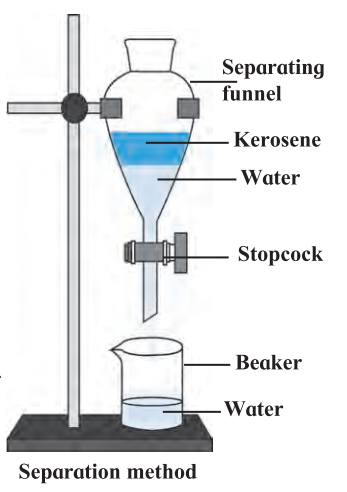
2. Separating funnel: When a mixture of two immiscible liquids is left undisturbed, two layers are clearly seen to have formed, (a) The heavier of the liquids remain below and the lighter liquid floats on it. (b) So two liquids in a mixture can be separated by making use of this property, e.g.
- to separate kerosene and water.
- to separate oil and water.

Question e.
Which precaution will you take while using the methods of distillation and separation by separating funnel?
Answer:
In using distillation:
- Allow sufficient space to work in, working area should be well lit and well ventilated to prevent the accumulation of alcoholic vapours.
- Keep a fire extinguisher handy.
- There should be no obstruction in the piping that could cause pressure build-up in the stills and cause it to burst.
In using separating funnel:
- Stopper should be tightly fitted so that the solution does not leak out when the separating funnel is inverted.
- Never throw any layer away until you are absolutely sure that you isolated the final product.
Project:
Question a.
Visit a jaggery or a sugar factory. Obtain information about the methods that are used to separate the components of the mixture while making jaggery or sugar. Present it in the class.
Class 7 Science Chapter 14 Elements, Compounds and Mixtures Important Questions and Answers
Fill in the blanks and rewrite the sentences:
Question 1.
………………, ……………….. and are three states of matter.
Answer:
Solid, liquid, gaseous
Question 2.
Molecules in ……………….. phase of matter are tightly packed together.
Answer:
solid

Question 3.
A ……………….. has a definite volume, but not a definite shape, it takes the shape of its container.
Answer:
liquid
Question 4.
The temperature at which a liquid becomes a solid is called ……………….. .
Answer:
freezing point
Question 5.
Anything that takes up space and has mass is called ……………….. .
Answer:
matter
Question 6.
A ……………….. has no definite shape and no definite volume.
Answer:
Gas
Question 7.
The amount of space that matter occupies is called ……………….. .
Answer:
volume

Question 8.
A ……………….. has a definite shape and definite volume.
Answer:
solid
Question 9.
When a liquid is heated it will ……………….. .
Answer:
expand
Question 10.
A ……………….. is formed by mixing different elements or compounds.
Answer:
mixture
Question 11.
The substance formed by a chemical combination of two or more elements is called a ……………….. .
Answer:
compound
Question 12.
The elements that show some properties of metals and non-metals are called ……………….. .
Answer:
metalloids

Question 13.
Elements are generally classified into ……………….. and ……………….. .
Answer:
metal, non-metals
Question 14.
The smallest particles of substances are called ……………….. .
Answer:
molecules
Question 15.
To date, scientists have discovered ……………….. elements and of these ……………….. elements occur in nature.
Answer:
118, 92
Question 16.
……………….. was the first scientist to use symbols for elements.
Answer:
Berzelius
Question 17.
Symbol for Tungsten is and it is ……………….. and it is derived from its German name ……………….. .
Answer:
W, Wolfram
Question 18.
22-carat gold is an ……………….. of gold.
Answer:
alloy

Question 19.
……………….. of a compound is a short form of its name written using symbols of its constituent elements.
Answer:
Molecular formula
Question 20.
For separating insoluble particles of blood (blood cells) from its liquid part (plasma), ……………….. method is used.
Answer:
centrifugation
Give scientific reason.
Question 1.
Saltwater is a mixture.
Answer:
- The salt and water are mixed to give saltwater and can both be separated.
- It can be separated by boiling saltwater.
- Water can be obtained by condensation whereas the salt will be leftover.
- As the salt and water do not react chemically and they are separated by simple processes therefore saltwater is called a mixture.

Question 2.
Classify the following substances according to their properties
(water, thermocol, soil, iron, coal, paper, rubber, copper, coir, plastic)
Answer:
| Metals | Mixtures | Compounds |
| Copper, Iron | Soil, Thermocol | Paper, Coir, Plastic, Rubber, Water |
Can you tell?
Answer the following questions:
Question 1.
What are objects made of?
Answer:
An object is made of a certain substance, (i) The term matter is also used as a synonym of substance, (ii) Object is made of matter.
Question 2.
What are these articles of everyday use made of?
Answer:
- Electric wire → copper-metal
- Kitchen utensils → stainless steel, Brass-alloy (aluminium-metal).
- Nails → iron-metal
- Tables, chairs → wood, plastic
- Sugar → sucrose
- Window panes → glass
- Salt → NaCl (Sodium chloride) it is a compound.

Question 3.
What do the short-forms Dr, H.M., AC, Adv., C.M., DC stand for?
Answer:
Short forms of Dr. – Doctor, H.M. – Headmaster, AC – Air conditioner, Adv – Advance, C.M. – Chief Minister, DC – Direct current
Question 4.
Which metals do we use in day-to-day life?
Answer:
- Gold and silver → used in jewellery, computer and solar cells
- Iron, copper, aluminium → used to make utensils
- Copper → used in electrical gadgets
- Tungsten → used in electric bulbs.
- Zinc → for coating on iron to prevent rusting
- Tin → for coating on copper and brass vessels
- Iron → nails, agricultural equipments, in construction of buildings, to make steel
Question 5.
Are metals elements?
Answer:
Yes, metals are elements.
Question 6.
Which element helps combustion?
Answer:
Oxygen helps combustion.

Question 7.
Does water help combustion?
Answer:
- Water is formed by a combination of hydrogen and oxygen but does not help combustion.
- It is used to extinguish a fire.
Question 8.
What are the mixtures used in everyday life?
Answer:
Oil and water, lemon juice and sherbet, bhel, honey and tea, milk and chocolate, coffee and cream, salt and water, smog (smoke + fog) air (oxygen + nitrogen), salad, milk.
Question 9.
Are all mixtures useful to us?
Answer:
No, adulterated foodstuff is also a kind of mixture and it is not useful. It is harmful, because when an unwanted and harmful substance is mixed with another substance the resulting mixture no longer remains useful.

Question 10.
How will you separate each component from a mixture of semolina, salt and iron filings?
Answer:
- If a magnet is moved through this mixture all iron filings will stick to the magnet, remove them.
- Add water to the remaining mixture, all salt will dissolve and then filter through filter paper.
- Samolina will settle in filter paper.
- The filtrate will be saltwater and salt can be separated by evaporation or distillation.
Use your brainpower!
Answer the following questions:
Question 1.
In day to day life we come across many things in our surroundings. We touch them, we study their properties. Are all these things made from only one kind of matter or from more than one kind of matters?
Answer:
- Things are made from only one kind of matter or may be made from more than one kind of matter.
- When a matter is made up of only one type of atoms it is called an element.
- We find many elements in our surroundings.
- But we also see many substances which are made up of two or more elements they are called compounds.
- Also we see many substances which are formed by physically mixing two or more elements, they are called mixtures.

Question 2.
Classify the following according to the nature of matter in them – whether it is made from one kind of matter or from more than one kind of matter, and whether it is in solid, liquid or gaseous state: an engraved idol, gold, milk, water, a plank, concrete, salt, soil, coal, smoke, sherbet, cooked khichadi, steam.
Answer:
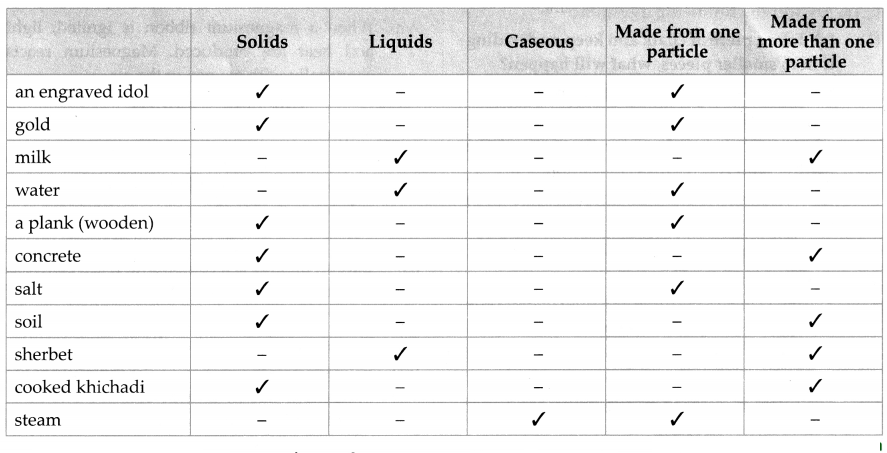
Question 3.
Which of the following are mixtures?
(water, sharbet, iron, steel, coal, air, salt, copper, brass, soil)
Answer:
Mixtures: sherbet, steel, air, brass and soil

Question 4.
Which elements are present in air?
Answer:
Nearly all of the earth’s atmosphere is made up of gases → Nitrogen, Oxygen, Carbon dioxide, Argon and Neon, Helium and Krypton.
Question 5.
Is carbon dioxide an element?
Answer:
No, carbon dioxide is a compound. A molecule of the compound CO
2
contains one atom of the element carbon and two atoms of the element oxygen.
Question 6.
What are the properties of elements due to?
Answer:
Properties of elements are due to molecules present in it. The molecules are made of one or more atoms which are exactly alike. The mass and volume of atoms of different elements are different.
Question 7.
Are the atoms of different elements similar or dissimilar?
Answer:
The atoms of different elements are dissimilar.

Question 8.
Is the water that falls from clouds naturally pure?
Answer:
- Any form of water that falls from the clouds is known as precipitation. Several forms → rain, snow, hail stones.
- Yes, it is pure as water vapours condense to form rain.
- The same process is used in distillation to obtain the purest water.
Question 9.
Which properties of a liquid are seen in the distillation method?
Answer:
Liquid when boiled turns into gaseous state (e.g. water vapours) and on cooling it condenses to liquid.
Question 10.
For what purposes is distilled water used?
Answer:
Distilled water is used in pharmacy as a solvent, in photography to wash the negatives, in laboratory experiments and in cleaning medical tools.
Answer the following questions:
Question 1.a.
Take a piece of chalk and keep on dividing it in to smaller pieces, what will happen?
Answer:
We will get very tiny particles of chalk.

Question b.
Wipe a drop of ink with a handkerchief what effect does it have on the cloth of the handkerchief?
Answer:
The cloth will absorb the ink drop and cloth will have a stain mark on it.
Question c.
What happens when the lid of a bottle of perfume is opened?
Answer:
The molecules of perfume move out of the bottle and collide with other molecules in the air and eventually perfume spreads throughout the room.
Question 2.
Fill water in a spray pump, spray the water and observe the spray.
Answer:
When we spray the water, spray is composed of small particles of water. They are very tiny that we can not see them.

Question 3.
Take water in a tea pot and cover it, heat the water to a boil. What do you see on the inside of the lid?
Answer:
- When we boil the water in a tea pot it boils and changes into gaseous state.
- We find vapours (steam) but when we cover it with a lid, the water vapour cools down, condenses into liquid. So we find water droplets on the inside of the lid.
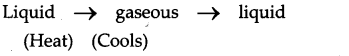
Question 4.a.
Take sugar in a test tube and heat the test tube. Observe what happens. What remains behind?
Answer:
When a test tube containing sugar is heated, the sugar melts and then it loses water leaving behind a black substance. This black substance is carbon.
Sugar is sucrose, a molecule of sugar is composed of 12 atoms of carbon, 22 atoms of hydrogen and 11 atoms of oxygen. (C
12
H
22
O
11
).
- Sugar is a compound made from these three elements carbon, hydrogen, oxygen.
- It is a Carbohydrate.

Question b.
Using tongs hold a magnesium ribbon in a flame and observe what changes took place?
Answer:
When a magnesium ribbon is ignited, light and heat are produced. Magnesium reacts chemically with oxygen in the air
2Mg
(s)
+ O
2(g)
→ 2MgO
(s)
Magnesium oxide is produced.
Question c.
What does the name carbon dioxide imply – how many and which elements is this substance made of?
Answer:
- Carbon dioxide is a compound. It is a tasteless, odourless gas. It is a natural product of respiration.
- Plants use this to produce food.
- Humans breath out the CO 2 .
[A molecule of the compound carbon dioxide contains one atom of the element carbon and two atoms of the element oxygen.]
Question d.
Which of these are compound, which are elements?
Answer:
- Water → compound H 2 O (hydrogen + oxygen), Oxygen → Element
- Carbon dioxide → compound CO 2 (Carbon + Oxygen)

Question e.
What is the smallest particle of a compound called?
Answer:
The smallest particle of a compound is called molecule.
Question 5.
From the internet or reference books obtain information about elements and prepare a table according to the format given below:
Answer:
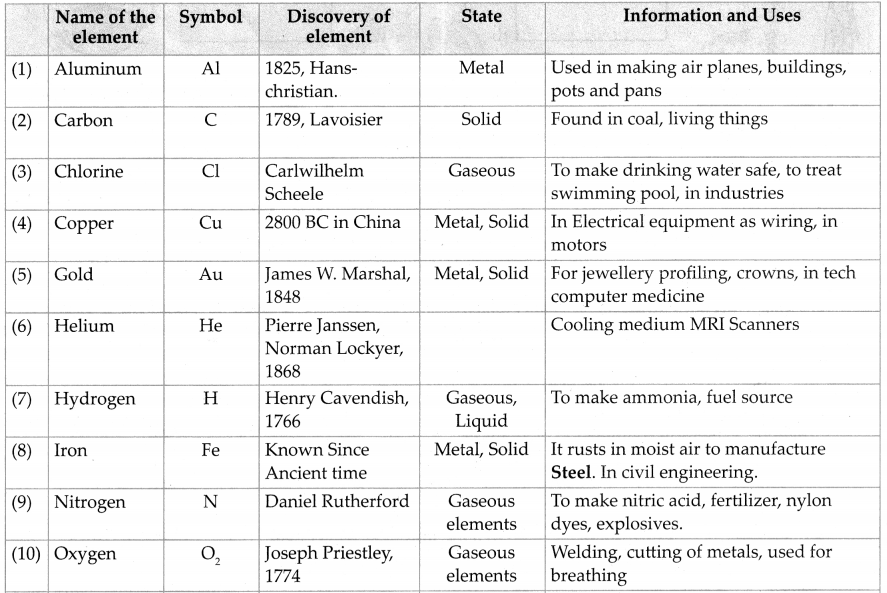
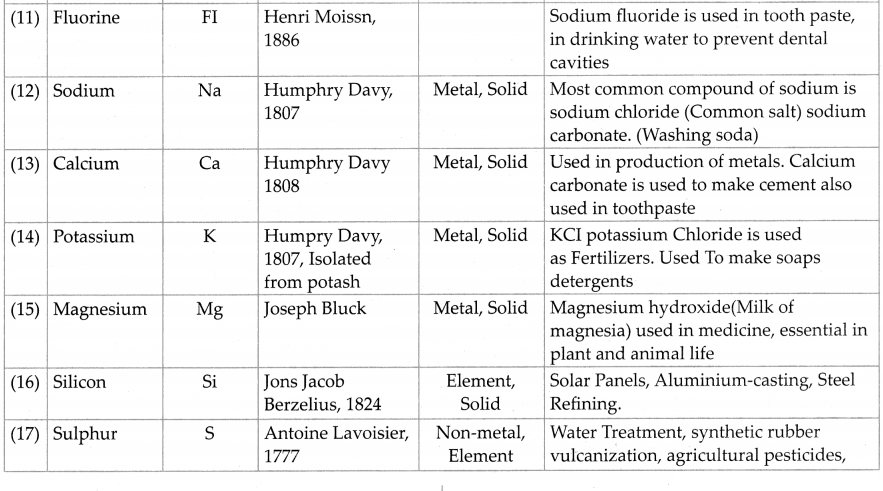

Question 6.
Some methods of separating the components of a mixture.
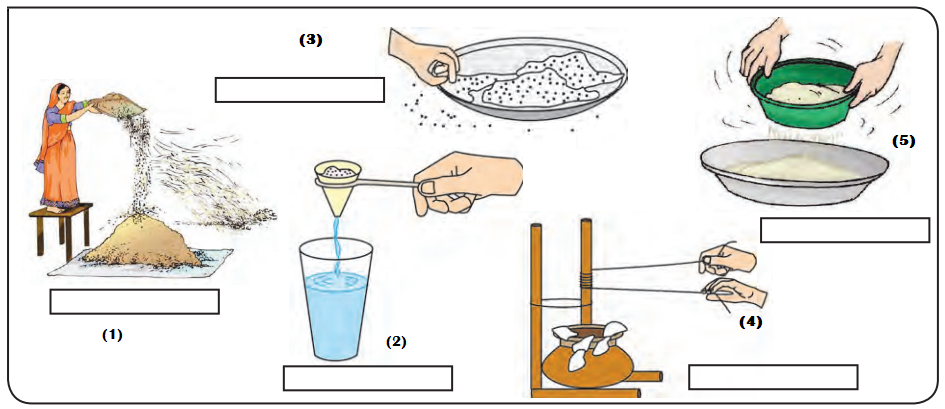
Answer:
- Winnowing
- Filteration
- Hand picking
- Churning
- Sieving
Question 7.
What is a molecular formula?
Answer:
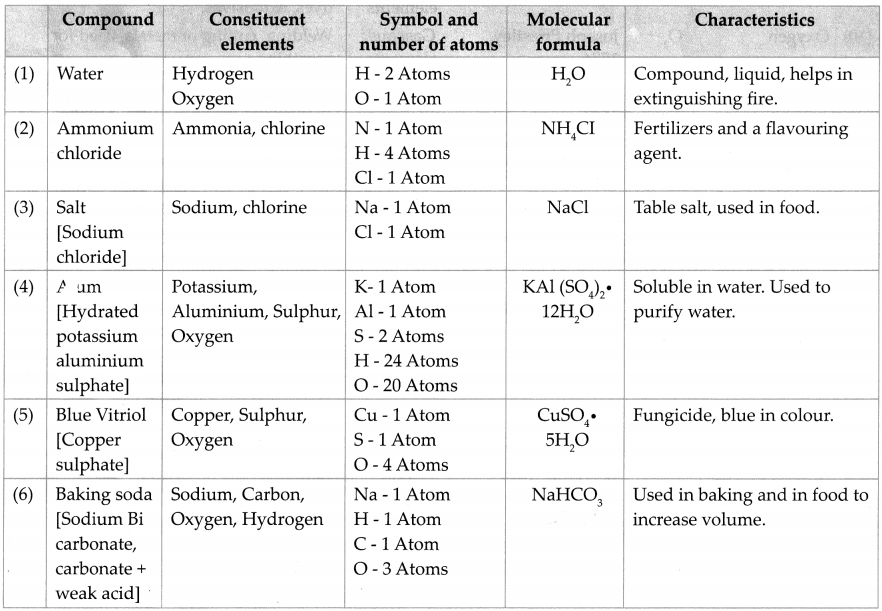
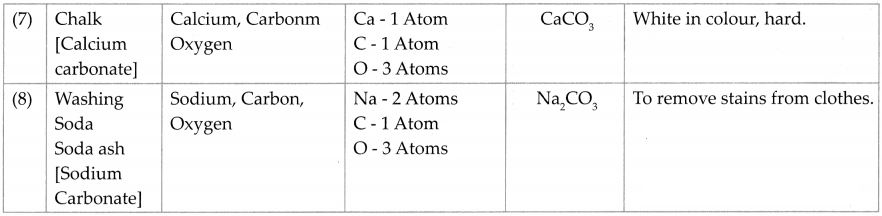
Like an element, a compound is also written in an abridged form. A molecule of a compound is formed by a chemical combination of atoms of two or more elements. Therefore a molecular formula is used to represent a compound. Molecular formula of a compound is a short form of its name written with the help of the symbols of constituent elements and the number of their respective atoms, e.g.: Nael – Sodium chloride.

Question 8.
Collect information and prepare table:
Write short notes on:
Question a.
Atoms
Answer:
Atoms are basic building blocks of matter. Desk, chair, air are made up of atoms.

Question b.
Compounds
Answer:
1. A compound is a pure substance that contains two or more elements. Compounds are chemical combination of elements with properties that are different from the elements that formed them.
2. Substance is a compound only if its molecules are made up of atoms of different types e.g. Water, one molecule of water is made of two atoms of hydrogen and one atom of oxygen.
Question c.
Mixtures
Answer:
- A mixture is two or more elements or compounds which are combined physically but no chemical reaction occurs.
- This means you can separate them again, e.g. air, brass.
Question 9.
Write short notes on:
(1) Distillation (2) Sublimation (3) Magnetic separation (4) Sedimentation (5) Filtration (6) Evaporation
Answer:
1. Distillation: It is a process of heating a solution containing soluble solids to form vapours of the liquid and then cooling the vapours to get the liquid back. e.g. A mixture of common salt and water is taken in distillation flask and heated. Steam rises up and comes out into condenser.
2. Sublimation: It is a process in which some solids on heating are transformed directly to vapour without passing through the liquid state, (i) It is used to separate a mixture of solids. The vapours are cooled separately, (ii) Used to separate ammonium chloride, iodine, camphor and sulphur from any mixture.
3. Magnetic separation: A mixture with iron fillings as one of the components can be separated using magnet to attract the iron particles away from the mixture.
4. Sedimentation: (i) It is a process of separating an insoluble solid from a liquid in which it is suspended, by allowing it to settle to the bottom of the container, e.g. Muddy water contains heavier particles like sand and soil, (ii) Leave this muddy water undisturbed for some time, (iii) The heavier soil and sand particles settle down and the clear upper water is poured out by decantation.
5. Filtration: It is used for separating insoluble solids from a liquid, e.g. mixture of chalk and water is poured through a filter paper in a funnel while the water gets collected in the beaker below, chalk is retained in the filter paper.
6. Evaporation: It is used for recovering dissolved solid substances from solutions by evaporation e.g. sugar can be recovered from sugar-water.

Question 10.
Name the method you will use to separate the following mixtures:
- Cream from buttermilk.
- Mud from muddy water.
- Stones from grains.
- Tea leaves from boiled tea.
- Salt from water.
- Kerosene and water.
- Blood cells and plasma.
- Ink from water
Answer:
- Churning
- Sedimentation and decantation
- hand picking
- filtration
- distillation
- separating funnel
- Centrifugation
- Chromatography

Question 11.
Draw the experimental setup of weperation using separating funnel:
Answer: