Balbharti Maharashtra State Board Class 9 Science Solutions Chapter 14 Substances in Common Use Notes, Textbook Exercise Important Questions and Answers.
Std 9 Science Chapter 14 Substances in Common Use Question Answer Maharashtra Board
Class 9 Science Chapter 14 Substances in Common Use Question Answer Maharashtra Board
1. Fill in the blanks.
a. The number of molecules of water of crystallization in washing soda is …………….…. .
b. The chemical name of baking soda is …………….…. .
c. …………….…. is used in treatment of hyperthyroidism.
d. The chemical name of Teflon is …………….…. .
Answer:
a. 10
b. Sodium bicarbonate
c. Iodine -123
d. Polytetra fluoroethylene (C
2
F
4
)
n

2. Match the pairs
| Group A | Group B |
| 1. Saturated brine | a. sodium metal freed |
| 2. Fused salt | b. basic salt |
| 3. CaOCl 2 | c. crystallization of salt |
| 4. NaHCO 3 | d. oxidation of colour |
Answer:
(1 – c),
(2 – a),
(3 – d),
(4 – b)
3. Write answers to the following
a. What is meant by radioactivity?
Answer:
- Elements with a high atomic number such as uranium, thorium, radium have a property of spontaneously emitting invisible, highly penetrating and high energy radiation.
- This property is called radioactivity.
- A substance having this property is called a radioactive substance.
b. When is said to be the nucleus unstable?
Answer:
- It is the balance of protons and neutrons in a nucleus which determines whether a nucleus will be stable or unstable.
- Too many neutrons or protons upset this balance disrupting the binding energy from the strong nuclear forces making the nucleus unstable.
c. Which diseases are caused by artificial food colours?
Answer:
Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
d. Where in the industrial field is radioactivity used?
Answer:
Industrial field Radiography
- Internal cracks and voids in cast iron articles and iron solder can be detected with the help of gamma rays.
- For this purpose, isotopes like cobalt-60, iridium-192 are used in the radiography camera.
- This technique is used for detecting flaws in metal work.
Measurement of thickness, density and level
- It is necessary to maintain the required thickness in the manufacture of aluminium, plastic, iron sheets of differing thickness.
- In the manufacturing process, a radioactive substance is placed on one side and an instrument to measure radiation on the other.
- The radiation read by the measuring instrument varies with the thickness of the sheet.
- Material inside a packing can also be examined by the same technique.
Luminescent paint and radio luminescence:
- The radioactive substances radium, promethium, tritium with some phosphor are used to make certain objects visible in the dark, for example, the hands of a clock, and certain other objects.
- Krypton-85 is used in HID (High-Intensity Discharge) lamps while promethium – 147 is used in portable X-ray units as the source of beta rays.
Use in Ceramic articles:
- Luminous colours are used to decorate ceramic tiles, utensils, plates, etc.
- Earlier uranium oxide was used in these paints.
e. Write down properties of teflon.
Answer:
Properties of teflon :
- The atmosphere and chemical substances have no effect on Teflon.
- Neither water nor oil will stick to Teflon coated articles.
- High temperatures do not affect Teflon as its melting point is 327 °C.
- Teflon coated articles are easy to clean.
f. What type of colours will you use to celebrate ecofriendly Rang Panchami? Why?
Answer:
- We regularly use artificial colours on Rang Panchami.
- The red colour used on Rang Panchami is very dangerous. It contains a high proportion of mercury in it.
- This poses risks like blindness, skin cancer, asthma, itching of the skin, permanent blocking of sweat pores, etc.
- Therefore, it is necessary to use eco-friendly colours.
- We will prepare colours for Rang Panchami from natural resources such as beet root, flowers of flame of forest, spinach, flame tree (gulmohar) and protect your health by using these.
g. Why has the use of methods like Teflon coating become more common?
Answer:
The use of methods like Teflon coating became more common because of following properties of teflon:
- The atmosphere, rain water and chemical substances have no effect on Teflon.
- Neither water nor oil will stick to Teflon coated articles.
- High temperatures do not affect Teflon as its melting point is 327 °C.
- Teflon coated articles are easy to clean.
- Teflon is a poor conductor of electricity.
4. Give a scientific explanation
a. Bleaching powder has the odour of chlorine.
Answer:
- Bleaching powder undergoes slow decomposition due to the carbon dioxide in air and chlorine gas is released.
-
Bleaching powder gets its property because of this release of chorine gas.

b. The hard water of a well becomes soft on adding washing soda to it.
Answer:
- The hard water from a well or a bore-well becomes soft on adding washing soda and we come to know this from the lather formed on it.
- The hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium in it.
- Na 2 CO 3 is added to it to soften such water and make it suitable for use.
-
The reaction with Na
2
CO
3
causes the formation of insoluble carbonate salts of magnesium and calcium.
MgCl 2(aq) + Na 2 CO 3(s) → MgCO 3(s) + 2 NaCl (s)
c. Soap forms a precipitate in hard water.
Answer:
- When soap is mixed with hard water, calcium and magnesium salts of fatty acids are formed.
- These being water insoluble, they form a precipitate and that is why lather is not formed.
d. The particles of powder are given an electric charge while spraying them to form the powder coating.
Answer:
- Powder coating is a method of applying a layer harder than paint on the surface of an iron object to prevent rusting.
- In this method, a polymer resin, a pigment and some other ingredients are melt, mixed, cooled and ground into a uniform powder.
- This powder is sprayed on the polished metal surface by electrostatic spray deposition (ESD).
- In this method, the particles of the powder are given an electrostatic charge due to which a uniform layer of the powder sticks to the metal surface.
- Then the object is heated in the oven along with the coating.
- A chemical reaction occurs in the layer, resulting in the formation of long cross-linked polymeric chains.
e. The aluminium article is used as an anode in the anodising process.
Answer:
- A protective layer is formed naturally on the surface of aluminium metal by reaction with oxygen in air.
- In the anodizing process, this layer is made of the desired thickness. Anodizing is done by electrolysis.
- Dilute Sulphuric acid is taken in the electrolytic cell and the aluminium article is dipped in it as the anode.
- When an electric current is passed, hydrogen gas is released at the cathode and oxygen gas at the anode.
- A reaction with oxygen occurs and a layer of hydrated aluminium oxide is formed on the anode. Therefore, aluminium article is used as an anode in the anodizing process.
f. When the radiation coming out from certain radioactive substance is passed through an electric field, marks are found at three places on the photographic plate placed in its path.
Answer:
(i) When the radiation coming out from certain radioactive substance is passed through an electric field, marks are found at three places on the photographic plate placed in its path. This is because the radiation coming out from radioactive substance are of three types Alpha rays, Beta rays and Gamma rays.
(ii) The rays which get deviated slightly towards negatively charged plate are called alpha rays. They get deviated towards negatively charged plate because they are made of positively charged particles, called as alpha particles (He++).
(iii) The rays which get deviated substantially towards the positively charged plate are called beta rays. They get deviated towards a positively charged plate because they are made of negatively charged particles called as beta particles (e-).
(iv) The rays which do not deviate at all are called gamma rays. They are uncharged electromagnetic radiation. Hence, marks are found at three places on the photographic plate. (For diagram refer Fig. 14 .2 from Q. 4(3))
g. A certain type of ceramic tiles are fixed on the outer layer of a space shuttle.
Answer:
(i) Each shuttle is covered by more than 24,000 of the six- by six-inch advance ceramic tiles. This tiles withstand high temperatures without decomposing. They are brittle, water-resistant and electrical insulator. Most of the tiles are made of silica fibres, which are produced from high-grade sand (SiO
2
).
(ii) Silica is an excellent insulator because it transports heat slowly. When the outer portion of a tile gets hot, the heat takes a long time to work its way down through the rest of the tile to the shuttle’s skin. The tiles keep the orbiter’s aluminium skin at 350 degrees or less.
(iii) The silica fibers are mixed with water and chemicals, and the mixture is poured into molds, which are zapped in microwave ovens at 2,350°C to fuse the silica fibres. Tiles are too brittle to attach to the orbiter directly.
(iv) The shuttle’s skin contracts slightly while in orbit, then expands during re-entry.
(v) In addition, the stresses of launch and re-entry cause the skin to flex and bend. Such motions could easily crack the tiles or shake them off. To keep them in place, workers glue the tiles to flexible felt-like pads, then glue the pads to the orbiter.
5. Write answers to the following
a. Write about artificial food colours, the substances used in them and their harmful effects.
Answer:
(a) Artificial food colour and substances in them.
- Food colours are mixed in most soft drinks and foodstuffs available in the market.
- These food colours are in the form of powders, gels and pastes.
- Food colours are used in domestic as well as commercial products.
- Certain colours and essences are added to ice cream, ice candies, sauce, fruit juices, cold drinks, pickles, jams and jelly.
- Food colours are often found to be added to packaged meat (chicken, mutton), chilli powder, turmeric, sweets and other similar substances so as to give them a good colour.
- Tetrazene, sunset yellow are artificial food colours used extensively.
(b) Harmful effects of artificial food colours
- Food colours added to pickles, jam and sauce contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis.
- Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
b. What is meant by water of crystallization? Give examples of salts with water of crystallization, and their uses.
Answer:
The exact number of water molecules which are chemically bonded to a molecule of a salt within a hydrated crystalline compound is called as water of crystallization are:
Some substances in our daily use which contain water of crystallization are:
- Alum (Potash alum – K 2 SO 4 .Al 2 (SO 4 ) 3 .24H 2 O)
- Borax (Na 2 B 4 O 7 .10H 2 O)
- Epsom salt (Magnesium sulphate MgSO 4 .7H 2 O)
- Barium chloride (BaCl 2 .2H 2 O)
- Sodium sulphate (Glauber’s salt Na 2 SO 4 .10 H 2 O)
- Blue vitriol (Copper Sulphate – CuSO 4 .5H 2 O)
Uses of these salts are as given below:
(i) Alum (Potash alum – K
2
SO
4
.Al
2
(SO
4
)
3
.24H
2
O)
- Alum is used in the process of water purification.
- Because of the property of coagulation, the solid impurities in water come together, become heavy and settle to the bottom. As a result, the impure water or muddy above becomes clear.
- Alum powder, found in the spice section of many grocery stores, may be used in pickling recipes as a preservative to maintain fruit and vegetable crispness.
- Alum is used as the acidic component of some commercial baking powders.
- Alum has been used as an after shave treatment.
(ii) Borax (Na 2 B 4 O 7 .10H 2 O)
- Borax today is used for many cleaning purposes.
- It is used to make homemade laundry detergent.
- It is added as a cleaning boost to any other detergent.
- It even keeps ants and other pests away.
(iii) Epsom salt (Magnesium sulphate MgSO 4 .7H 2 O)
- Epsom salt is used as a relaxing magnesium bath soak.
- To grow better vegetables – Add a tablespoon of Epsom salt to the soil below a vegetable plant to boost growth.
(iv) Barium chloride (BaCl 2 .2H 2 O)
- In industry, Barium chloride is mainly used in the purification of brine solution in caustic chlorine plants.
- It is also used in the manufacture of heat treatment salts.
- It is used in hardening of steel.
- It is used in the manufacture of pigments.
(v) Sodium sulphate (Glauber’s salt Na 2 SO 4 .10H 2 O)
- In the laboratory, anhydrous Sodium sulphate is widely used as an inert drying agent, for removing traces of water from organic solutions.
- Glauber’s salt, the decahydrate, is used as a laxative.
(vi) Blue vitriol (Copper sulphate CuSO 4 .5H 2 O)
- Blue vitriol is used in the blood test for diagnosing anaemia.
- Slaked Time is used with blue vitriol in the Bordeaux mixture which is used as a fungicide on fruits like grapes and musk melon.
c. Write briefly about the three methods of electrolysis of sodium chloride.
Answer:
- When an electric current is passed through a saturated solution of sodium chloride (brine) it is electrolysed and hydrogen gas is released at the cathode while chlorine gas is released at the anode.
- This method is used for production of chlorine gas. In this method an important basic compound NaOH is formed in the cell.
-
Chemical reaction
2NaCl + 2H 2 O → 2NaOH + Cl 2 ↑ + H 2 ↑ - When salt is heated to a high temperature (about 800 °C), it melts. This is called the fused state of the salt.
6. Write the uses.
a. Anodizing
b. Powder coating
c. Radioactive substances
d. Ceramic
Answer:
(a) Anodizing : Anodizing is done on aluminum cooking utensils like griddles and cookers.
(b) Powder coating : Powder coating is done on iron objects to prevent rusting. Also, on Plastic and Medium density fibre (MDF) board, to make them highly durable, hard and attractive.
(c) Radioactive substances:
(I) Industrial field:
(i) Industrial Radiography : Internal cracks and voids in cast iron articles and iron solder can be. detected with the help of gamma rays. For this purpose, isotopes like cobalt-60, iridium-192 are used in the radiography camera. This technique is used for detecting flaws in metal work.
(ii) Measurement of thickness, density and level: It is necessary to maintain the required thickness in the manufacture of aluminium, plastic, iron sheets of differing thickness. In the manufacturing process, a radioactive substance is placed on one side and an instrument to measure radiation on the other. The radiation read by the measuring instrument varies with the thickness of the sheet. Material inside a packing can also be examined by the same technique.
(iii) Luminescent paint and radioluminescence:
The radioactive substances radium, promethium, tritium with some phosphor are used to make certain objects visible in the dark, for example, the hands of a clock, and certain other objects. Krypton-85 is used in HID (High Intensity Discharge) lamps while promethium-147 is used in portable X-ray units as the source of beta rays.
Use in Ceramic articles – Luminous colours are used to decorate ceramic tiles, utensils, plates, etc. Earlier uranium oxide was used in these paints.
(II) Field of agriculture :
- The genes and chromosomes that give seeds properties like fast growth, higher productivity, etc. can be modified by means of radiation.
- The radioactive isotope cobalt-60 is used for food preservation.
- Onions, potatoes are irradiated with gamma rays from cobalt-60 to prevent their sprouting.
- Strontium-90 is used as a tracer in the research on various crops.
(III) Medical science:
- Polycythemia : The red blood cell count increases in the disease polycythemia. Phosphorus- 32 is used in its treatment.
- Bone cancer : Strontium-89, strontium-90, samarium-153 and radium-223 are used in the treatment of bone cancer.
- Hyperthyroidism : Enlargement of thyroid gland, weight loss in spite of appetite, insomnia are the symptoms of hyperthyroidism.
- It occurs due to overproduction of hormones by the thyroid gland. Iodine-123 is used in the treatment of hyperthyroidism.
- Tumour detection : Boron-10, iodine-131, cobalt-60 are used in treatment of brain tumour, while arsenic-74 is used in detection of small tumours in the body.
(d) Ceramic : Pots made by a potter, Mangalore roofing tiles, construction bricks, pottery, terracotta articles are some examples of common ceramic articles that we see around.
7. Write the harmful effects
a. Artificial dye
b. Artificial food colour
c. Radioactive substances
d. Deodorant
Answer:
(a) Artificial dye:
- Dyeing hair can have adverse effects like hair fall, damage to hair texture, burning of skin, adverse effect on eyes, etc.
- Lipstick contains a dye named carmine. It does not affect lips but causes stomach disorders.
- Excessive use of plants for making natural dyes results in deterioration of the environment.
(b) Artificial food colour:
- Food colours added to pickles, jams and sauces contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis.
- Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
(c) Radioactive substances :
- The central nervous system is affected by radioactive radiations.
- Hereditary defects are generated by bombardment of radiation on D.N.A in the body.
- Radioactive radiation can penetrate the skin, and causes diseases like skin cancer, leukemia.
- The radioactive pollutants created due to explosions enter the body through air and it is difficult to control them.
- The radioactive pollutants released in the sea enter the bodies of fishes and through them enter the human body.
- The radioactive paint on the watch can cause cancer.
- The radioactive isotopes strontium-90 can enter the body through plants, fruits, flowers, cereals, milk, etc. and cause diseases like bone cancer, leukemia.
(d) Deodorant:
- Aluminium – Zirconium compounds are the most harmful chemicals in the deodorant. Disorders like headache, asthma, respiratory disorders, heart disease are likely to occur without our knowledge.
- There is a possibility of various skin disorders and also skin cancer due to the aluminium chlorohydrates.
8. Write the chemical formula Bleaching powder, common salt, baking soda, washing soda
Answer:
| Common name | Chemical name |
| Bleaching powder | CaOCl 2 |
| Common salt | NaCl |
| Baking soda | NaHCO s |
| Washing soda | Na 2 CO 3 .10 H 2 O |
9. Explain what you see in the following picture

Powder coating
Answer:
- This picture shows powder coating of the given object. Powder coating is a method of applying a layer harder than paint on the surface of an iron object to prevent rusting.
- In this method, a polymer resin, a pigment and some other ingredients are melt mixed, cooled and ground into a uniform powder.
- This powder is sprayed on the polished metal surface by electrostatic spray deposition (ESD).
- In this method, the particles of the powder are given an electrostatic charge due to which a uniform layer of the powder sticks to the metal surface.
- Then the object is heated in the oven along with the coating.
- A chemical reaction occurs in the layer, resulting in the formation of long cross-linked polymeric chains.
- This powder coating is highly durable, hard and attractive. Powder coating can be done on plastic and medium density fibre (MDF) board in day to day use as well.
Class 9 Science Chapter 14 Substances in Common Use Intext Questions and Answers
Can you recall?
Question 1.
What are detergents?
Answer:
- Detergent is a water-soluble cleansing agent which combines with impurities and dirt to make them more soluble, and differs from soap in not forming a scum with the salts in hard water.
- Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they’re generally liquids rather than solids.
- Detergents are used in everything from hair shampoo and clothes, washing powder to shaving foam and stain removers.
- The most important ingredients in detergents are chemicals called surfactants – a word made from bits of the words surface-active agents.
Question 2.
What are the important substances that we use in day to day life? For what purposes do we use them?
Answer:
Some of the substances we use in our day to day life and the purpose of using them are as follows:
Toothpaste: for cleaning teeth and maintaining hygienic conditions of mouth.
Soap : for bathing and maintaining hygienic conditions of body.
Natural and artificial fibers : we wear them as clothes to protect our body and many other purposes.
Washing powder: for cleaning clothes. Vegetables, fruits and food grains : they provide us with necessary vitamins, minerals and carbohydrates required for proper functioning of body.
Water : very important for our survival and many other functions of day to day life.
Metals : for making buildings, bridges, roads, vehicles, trains, ships, airplanes, utensils etc.
Acids, bases and salts : used for various purposes, for example, Sodium chloride which is used as common salt, a very necessary ingredient of our daily food without which our food is tasteless.
Question 3.
How are the various substances in day to day use classified from the scientific point of view?
Answer:
The various substances in day to day use are classified from the scientific point of view as elements, compounds, mixtures, metals, non-metals, acids, bases and salts.
Question 4.
Which chemicals and apparatus will you use in the laboratory for making soap?
Answer:
For making soap in laboratory following chemicals and apparatus are used :
Chemicals : Vegetable oil (coconut oil, castor oil, soya bean oil etc.)
Sodium hydroxide (NaOH)
Salt (Sodium chloride NaCl)
Apparatus :
500 ml glass beaker
Burner
Flat glass tray
Knife
Soaps are essential to personal and public health. They safely remove germs, soils and other contaminants and help us to stay healthy and make our surroundings more pleasant. Soaps are made from fats and oils or their fatty acids.
Take about 20 ml of any vegetable oil (coconut oil, castor oil, soybean oil etc.) in a beaker. Put about 30 – 35 ml of NaOH in it and heat and stir the mixture till a paste is formed. Now add a little salt. Stir again and allow the mixture to cool in a flat glass tray, on cooling it solidifies. Take a knife and cut it in soap bars as per the size required.
Question 5.
What are salts?
Answer:
- Inorganic substances occur naturally in the form of salts rather than acids or bases.
- The ionic compounds which do not contain H+ and OH ions and contain only one kind of cation and anion are called simple salts.
- For example, Na 2 SO 4 , K 3 PO 4 , CaCl 2 .
- The sea is said to be a rich source of salts. In fact, the sea is a rich source of several salts of various elements such as chlorine, sodium, magnesium, potassium, calcium, bromine.
- The important salts found in sea water are sodium chloride, magnesium chloride, magnesium sulphate, potassium chloride, calcium carbonate and magnesium bromide.
Answer the following questions:
Question 1.
How is it determined whether a substance is acidic, basic or neutral?
Answer:
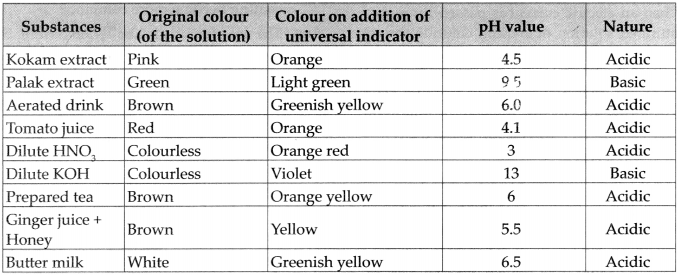
- In the pH scale, the pH of solutions varies from 0 to 14 in accordance with the strength of the acids or base.
- The pH scale shows different colours at different values of pH. Colour from dark red to yellow or from pH value 0 to 6 indicates the substances are acidic.
- Colour from light blue to violet or from pH value 8 to 14 indicates the substances are basic.
- Green colour or pH value 7 indicates the substances are neutral.
Question 2.
A sweets shop looks attractive because of the colourful sweets displayed there. Which colours are used in these substances?
Answer:
- Food colours and essences are used in sweets to make them colourful and tasty.
- These food colour are in the form of powders, gels and pastes.
- Food colours are natural as well as artificial.
- The food colours prepared from seeds, beetroot, flowers and fruit concentrate are natural.
- Tetrazene, sunset yellow are artificial food colours used extensively.
Question 3.
Make a list of substances in day to day use in accordance with their pH value (O to 14).
Answer:
Question 4.
A doctor gives you medicines when you fall ill. What are the medicines made from?
Answer:
- Long before pure chemicals were manufactured in labs, people used plants for medicine.
- There are over a hundred active ingredients derived from plants for use as drugs and medicines.
- The emergence of synthetic chemistry in the 1800s opened up new avenues for scientific research into drugs.
- Many chemists tried to locate medically active ingredients in plants (for instance the important malaria remedy quinine), and subsequently tried to make those substances in the laboratory in order to become independent of plant supplies.
- At the same time, a new industry developed as scientists developed processes to synthesise new chemicals on a large scale.
- New production methods developed in the late 1800s and early 1900s made it easier to standardise, package and transport medicines.
Question 5.
What problems do you get after playing colours on Rang Panchami? Why?
Answer:
- After playing colours on Rang Panchami we may get problems such as risks of blindness, skin cancer, asthma, itching of the skin, permanent blocking of sweat pores etc.
- This is because the colours used in rang panchami specially the red colour is very dangerous.
- It contains a high proportion of mercury in it.
Question 6.
Which colours will you use to prevent the occurrence of these problems?
Answer:
- To prevent the occurrence of these problem, instead of using artificial colours, natural colours should be used.
- Natural colours are prepared from natural resouroes such as beet root, flowers of flame of forest, spinach, flame tree (gulmohar).
Question 7.
What problem do you have on painting the house and furniture?
Answer:
- On painting the house and furniture with artificial colours which are dangerous, we may get problems such as risks of blindness, skin cancer, asthma, itching of the skin, permanent blocking of sweat pores, etc.
- This is because the colours contain a high proportion of mercury in it.
Question 8.
What is the property of Teflon because of which it is used in non-stickware?
Answer:
Following are the properties of teflon because of which it is used in nonstick cookware.
- The atmosphere and chemical substances have no effect on Teflon.
- Neither water nor oil will stick to Teflon coated non-stickware.
- High temperatures do not affect Teflon as its melting point is 327 °C.
- Teflon coated non-stickware are easy to clean.
Answer the following questions:
Question 1.
What is the strip shown below? What is it used for?
Answer:
The strip shown is pH metre scale. It is used to determined pH of solutions.
Question 2.
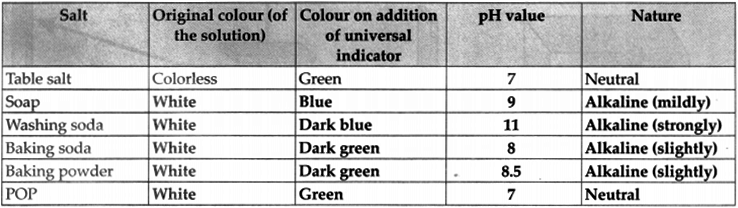
Prepare saturated solutions of given salts and put 2-3 drops of the universal indicator in them and note your observations in the table below:
Answer:
Class 9 Science Chapter 14 Substances in Common Use Additional Important Questions and Answers
Select the correct option:
Question 1.
Baking soda is ………………………… .
(a) non-metallic
(b) metallic
(c) acidic
(d) basic
Answer:
(d) basic
Question 2.
During electrolysis of NaCl in the fused state ………………………… is formed at cathode.
(a) sodium
(b) oxygen
(c) oxalic acid
(d) butyric acid
Answer:
(a) sodium
Question 3.
………………………… is known as chloride of lime.
(a) Calcium carbonate
(b) Sodium bicarbonate
(c) Sodium chloride
(d) Bleaching powder
Answer:
(d) Bleaching powder
Question 4.
Salt obtained from certain type of rock is called ………………………… .
(a) hard salt
(b) rock salt
(c) stone salt
(d) pure salt
Answer:
(b) rock salt
Question 5.
The ………………………… % aqueous solution of salt is called brine.
(a) 25
(b) 60
(c) 30
(d) 50
Answer:
(a) 25
Question 6.
The molecular formula for baking soda is ………………………… .
(a) Na
2
CO
3
(b) NaHCO
3
(c) Na
2
SO
4
(d) NaNO
3
Answer:
(b) NaHCO
3
Question 7.
Bleaching powder is also called ………………………… .
(a) Calcium oxygen chlorine
(b) Calcium oxychloride
(c) Calcium hydroxide
(d) Calcium oxalate
Answer:
(b) Calcium oxychloride
Question 8.
NaHCO
3
is also called as ………………………… .
(a) Sodium carbonate
(b) Sodium bicarbonate
(c) Sodium carbon oxide
(d) Sodium chloride
Answer:
(b) sodium bicarbonate
Question 9.
Molecular formula for Magnesium bromide is ………………………… .
(a) MgBr
(b) Mg
2
Br
(c) Mg
2
Br
2
(d) MgBr
2
Answer:
(d) MgBr
2
Question 10.
The molten state of NaCl is also called as ………………………… .
(a) solid state
(b) semi-solid
(c) gaseous state
(d) fused state
Answer:
(d) fused state
Question 11.
Substance used to make hard water soft is ………………………… .
(a) Na
2
CO
3
.10H
2
O
(b) Na
2
CO
3
.H
2
O
(c) NaCO
3
.10H
2
O
(d) NaCO
3
.H
2
O
Answer:
(a) Na
2
CO
3
.10H
2
O
Question 12.
The fixed number of molecules of water in alum is ………………………… .
(a) 24
(b) 10
(c) 7
(d) 2
Answer:
(a) 24
Question 13.
Chlorine gas is a strong ………………………… agent.
(a) reducing
(b) oxidising
(c) neutral
(d) acidic
Answer:
(b) oxidising
Question 14.
Bleaching powder is ………………………… in colour.
(a) brownish red
(b) yellowish-white
(c) pinkish brown
(d) greenish blue
Answer:
(b) yellowish-white
Question 15.
Soap is ………………………… in nature.
(a) acidic
(b) basic
(c) neutral
(d) fuming
Answer:
(b) basic
Question 16.
Melting point of NaCl is ………………………… .
(a) 400°C
(b) 100°C
(c) 800°C
(d) 600°C
Answer:
(c) 800°C
Question 17.
Rock salt is ………………………… in colour.
(a) black
(b) white
(c) brown
(d) blue
Answer:
(c) brown
Question 18.
Strong odour in swimming pool is due to ………………………… .
(a) chlorine gas
(b) impurities
(c) calcium carbonate
(d) calcium hydroxide
Answer:
(a) chlorine gas
Question 19.
Molecular formula of sodium bicarbonate is ………………………… .
(a) NaHCO
3
(b) Na
2
HCO
3
(c) Na
2
HCO
2
(d) NaHCO
2
Answer:
(a) NaHCO
3
Question 20.
Soap is ………………………… in nature.
(a) radioactive
(b) natural
(c) acidic
(d) basic
Answer:
(d) basic
Question 21.
The molecular formula for Magnesium bromide is ………………………… .
(а) MgBr
(b) MgBr
2
(c) Mg
2
Br
2
(d) Mg
2
Br
Answer:
(b) MgBr
2
Fill in the blanks and rewrite the complete statements:
(1) 25% of NaCl is known as
(2) gas is released when bleaching powder is added to water.
(3) Washing soda is as it absorbs moisture if left exposed to air.
(4) is used to make hard water soft.
(5) Molecular formula for Alum is
(6) is used in blood test for diagnosing anaemia.
(7) High grade of and are used as raw material to manufacture bathing soap.
(8) Pickles, jams and sauce contain small quantities of and
(9) Excessive consumption of foods with added food colours leads to diseases like
(10) Melting point of Teflon is
(11) Anodizing is done by
(12) The red blood cell count increases in the disease
(13) Magnesium sulphate (MgS04.7H20) is also called
Answer:
(1) Brine
(2) Chlorine
(3) hygroscopic
(4) Na
2
CO
3
(Washing soda)
(5) K
2
SO
4
.Al
2
(SO)
3
.24H
2
O
(6) Blue Vitriol
(7) fats, oils
(8) lead, mercury
(9) ADHD (Attention Deficit Hyperactivity Disorder)
(10) 327°C
(11) Electrolysis
(12) Polycythemia
(13) Epsom salt
(14) Soap
State whether the following statements are true or false. Correct the false statements.
(1) Bleaching powder is used in preparation of chloroform which is used as an anaesthetic.
(2) On addition of universal indicator the colour of table salt changes to pink.
(3) Borax is Na
2
B
4
O
7
.9H
2
O.
(4) Boron -10 is used to cure bone cancer.
(5) Clinical deo contains 20 to 25% of aluminium.
(6) NaHCO
3
is sodium carbonate.
(7) Soap is acidic in nature.
(8) Baking powder is neutral in nature.
(9) POP is crystalline in nature.
(10) pH value of universal indicator is 0 – 7.
(11) Sodium chloride is formed by neutralization reaction between sodium hydroxide and calcium carbonate.
(12) Boiling point of NaCl is 800 °C.
(13) Molten state of NaCl is also called as solid state.
(14) Mineral halite is an example of Rock Salt.
(15) Sodium bicarbonate is used to reduce acidity.
(16) Bleaching powder is also called as chloride of lime.
(17) Bleaching powder is obtained by reaction of chlorine gas with slaked lime.
(18) Bleaching powder available in market is classified on the basis of colour.
(19) The hard water becomes soft on adding baking soda (NaHCO
3
)
Answer:
(1) True
(2) False. On addition of universal indicator the colour of table salt changes to green.
(3) False. Borax is Na
2
B
4
O
7
.9H
2
O
(4) False. Boron – 10 is used in treatment of brain tumour.
(5) True
(6) False. NaHCO
3
is sodium bicarbonate.
(7) False. Soap is basic in nature.
(8) False. Baking powder is basic in nature.
(9) False. POP is amorphous in nature.
(10) False. pH value of universal indicator is 0 -14.
(11) False. Sodium chloride is formed by a neutralization reaction between sodium hydroxide and hydrochloric acid.
(12) False. Melting point of NaCl is 800°C.
(13) False. Molten state of NaCl is also called as fused state.
(14) True
(15) True
(16) True
(17) True
(18) False. Bleaching powder available in market is classified on the basis of percentage of chlorine present in it.
(19) False. Hard water becomes soft on adding washing soda (Na
2
CO
3
.10H
2
O)
Name the following:
Question 1.
Important salts found in sea water.
Answer:
Magnesium chloride, Potassium chloride
Question 2.
Chemical used in preparation of breads and cakes to make them soft.
Answer:
Sodium bicarbonate
Question 3.
Gas liberated when bleaching powder is added to CO
2
gas.
Answer:
Chlorine (Cl
2
) gas.
Question 4.
Salt used in refining petroleum
Answer:
Sodium carbonate (Na
2
CO
3
)
Question 5.
Chemical formula of bleaching powder
Answer:
CaOCl
2
Question 6.
The chemical formula of Sodium hydrogen carbonate.
Answer:
NaHCO
3
Question 7.
Nature of soap.
Answer:
Basic
Question 8.
Molecular formula for Magnesium bromide
Answer:
MgBr
2
Question 9.
Melting point of NaCl
Answer:
800 °C
Question 10.
Two examples of Rock Salt
Answer:
Mineral halite and Himalayan rock salt.
Question 11.
Uses of sodium bicarbonate
Answer:
To prepare breads, cakes and dhokla
Question 12.
Constituents of baking powder.
Answer:
Baking soda and mild acid (tartaric acid)
Question 13.
Substance used to make hard water soft.
Answer:
Washing soda i.e. Na
2
CO
3
.10H
2
O.
Question 14.
Radioactive isotopes used in treatment of bone cancer.
Answer:
Strontium – 89, Strontium – 90, Samarium -153, Radium – 223
Question 15.
Use of Strontium 89 and strontium 90.
Answer:
Treatment of bone cancer.
Select the odd man out:
Question 1.
Sodium sulphate, Barium chloride, Magnesium sulphate, Bleaching powder.
Answer:
Bleaching powder, all others are crystalline salt with water of crystallization while bleaching powder is not.
Question 2.
Strontium – 89, Strontium – 90, Radium – 223, Iodine -123.
Answer:
Iodine -123, it is used to cure hyperthyroidism while others are used to cure bone cancer.
Question 3.
Soap, Toothpaste, Baking soda, Curd.
Answer:
Curd, it is acidic in nature while others are basic in nature.
Question 4.
Na
2
CO
3
.10H
2
O, Na
2
SO
4
.10H
2
O, BaCl
2
.2H
2
O, CaOCl
2
Answer:
CaOCl
2
, it is amorphous in nature while rest are crystalline in nature.
Question 5.
Boron-10, Iodine – 131, Cobalt – 60, Iodine – 123.
Answer:
Iodine -123, it is used in hyperthyroidism while rest are used in treatment of brain tumour.
Question 6.
Na
2
SO
4
, K
3
PO
4
, MgBr
2
, HCl.
Answer:
HCl, rest all are salts but HCl is an acid.
Write the correlated terms:
(1) Washing Powder : Basic :: Milk : ……………………………
(2) Na
2
SO
4
: Sodium sulphate :: K
3
PO
4
: ……………………………
(3) CaOCl
2
: Calcium Oxychloride : : NaHCO
3
: ……………………………
(4) Borax : 10H
2
O : : Alum : ……………………………
(5) Sodium Sulphate : Na
2
SO
4
.10H
2
O : : Magnesium Sulphate : ……………………………
(6) Bathing soap : High grades fats and oils :: Washing soap : ……………………………
(7) Radium – 23 : Bone cancer :: Phosphorus – 32 : ……………………………
(8) Iodine -123 : Hyperthyroidism :: Iodine -131 : ……………………………
(9) Clinical deo : 20-25% of aluminium : : Antiperspirant deo : of aluminium chlorohydrate : ……………………………
(10) Sodium carbonate : Na
2
CO
3
: : Sodium bicarbonate : ……………………………
(11) Curd : Acidic : : Soap : ……………………………
(12) Calcium Carbonate : CaCO
3
: : Magnesium bromide : ……………………………
(13) Baking Soda : NaHCO
3
: : Washing Soda : ……………………………
(14) Baking Soda : Breads and Cakes : : Washing Soda : ……………………………
(15) Borax : Na
2
B
4
O
7
.10H
2
O : : Alum : ……………………………
Answer:
(1) Acidic
(2) Potassium phosphate
(3) Sodium hydrogen carbonate
(4) 24H
2
O
(5) MgSO
4
.7H
2
O
(6) Low-grade fats and oils
(7) Polycythemia
(8) Tumour detection
(9) 15%
(10) NaHCO.
(11) Basic
(12) MgBr
2
(13) Na2CO
3
.10H
2
O
(14) Hard water soft
(15) K
2
SO
4
.Al
2
(SO
4
)
3
.24H
2
O
Match the columns:
Question 1.
| Column ‘A’ | Column ‘B’ | |
| (1) Barium chloride | (a) | 24H 2 O |
| (2) Sodium sulphate | (b) | 7H 2 O |
| (3) Magnesium sulphate | (c) | 2H 2 O |
| (4) Alum | (d) | 10H 2 O |
Answer:
(1 – c),
(2 – d),
(3 – b),
(4 – a)
Question 2.
| Column ‘A’ | Column ‘B’ |
| (1) Strontium – 90 | (a) Brain tumour |
| (2) Cobalt – 60 | (b) Hyperthyroidism |
| (3) Iodine – 131 | (c) Polycythemia |
| (4) Phosphorus – 32 | (d) Bone cancer |
Answer:
(1 – d),
(2 – a),
(3 – b),
(4 -c)
Give scientific reasons:
Question 1.
Baking soda is used while making cakes.
Answer:
- Baking soda is chemically known as sodium bicarbonate.
- On heating, sodium bicarbonate decomposes to form sodium carbonate, water and carbon dioxide (CO,).
- The carbon dioxide produced is released due to which the cake becomes soft and spongy.
- Hence, baking soda is used to make cake spongy and soft.
Question 2.
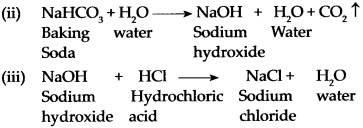
Baking soda is used to reduce acidity in the stomach.
Answer:
-
Baking soda is used as a medicine (antacid). When it is taken, it undergoes hydrolysis to give sodium hydroxide in the stomach. Thus, sodium hydroxide neutralizes the hydrochloric acid produced by gastric juice and gives relief to the patient from acidity.
Question 3.
Tap water and water in swimming pool has a typical strong odour in the rainy season.
Answer:
- Tap water and water in swimming pool has a typical strong odour in the rainy season, it is the odour of the chlorine gas that is used to destroy the microbes in the water.
- Chlorine gas is a strong oxidizing agent and therefore, it has a strong disinfecting as well as bleaching action.
- Chlorine is inconvenient to handle because of its gaseous state. Instead, the solid bleaching powder which has the same effect is more convenient to use.
-
Bleaching powder undergoes slow decomposition due to the carbon dioxide in air and chlorine gas is released. Bleaching powder gets its property because of this release of chlorine gas.

Question 4.
Sodium carbonate is added to hard water to make it soft.
Answer:
- The hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium in it.
- Na 2 CO 3 is added to it to soften such water and make it suitable for use.
-
The reaction with Na
2
CO
3
causes the formation of insoluble carbonate salts of magnesium and calcium thus making the water soft.
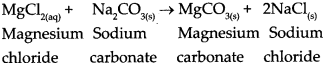
Question 5.
Alum is used in the process of water purification.
Answer:
- Alum is used in the process of water purification because of property of coagulation.
- The solid impurities in water come together, become heavy and settle to the bottom.
- As a result, the water above becomes clear.
Question 6.
Overconsumption of artificial food colours should be avoided?
Answer:
- The overconsumption of artificial food colours should be avoided because they can be detrimental to health.
- Food colours added to pickles, jam and sauce contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis.
- Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
Question 7.
Artificial colours in Rang Panchami should be used cautiously.
Answer:
- We regularly use artificial colours on Rang Panchami.
- The red colour used on Rang Panchami is very dangerous. It contains a high proportion of mercury in it.
- This poses risks like blindness, skin cancer, asthma, itching of the skin, permanent blocking of sweat pores etc.
- Therefore, it is necessary that artificial colours are used cautiously.
Question 8.
There are various brands of bleaching powder in the market.
Answer:
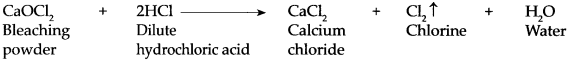
- When bleaching powder comes in contact with air, it reacts with carbon dioxide to form calcium carbonate and releases chlorine gas. CaOCl 2 + CO 2 → CaCO 3 + Cl 2 ↑ (from air)
- This generated chlorine is known as “available chlorine.”
- On the basis of the percentage of “available chlorine” various brands of bleaching powder are available in the market.
Question 9.
We use anodized cooking utensils like griddles and cookers.
Answer:
- Anodizing is process of forming a protective rayer of hydrated aluminium oxide on utensils.
- Due to anodizing, the surface of the cooking utensils become non-standing, non-toxic and non-reactive with the ingredients of the foot.
- These utensils get heated faster and withstand high heat.
- Also the amount of butter, ghee or oil used to cook food in these type of utensils is neglible.
- These utensils are tough, durable and resistant to corrosion.
- Hence, we use anodized cooking utensils like gridders and cookers.
Explain the following chemical reactions with the help of balanced equations :
Question 1.
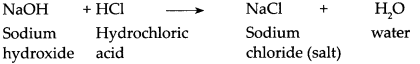
Sodium hydroxide reacts with dilute Hydrochloric acid.
Answer:
When Sodium hydroxide reacts with dilute Hydrochloric acid, it forms sodium chloride and water.
This is a neutralization reaction.
Question 2.
When an electric current is passed through a saturated solution of sodium chloride (brine)
Answer:
When an electric current is passed through a saturated solution of sodium chloride (brine) it is electrolysed and hydrogen gas is released at the cathode while chlorine gas is released at the anode. Also, an important basic compound NaOH is formed in the cell.
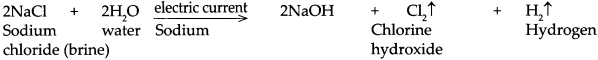
Question 3.
Baking soda (Sodium hydrogen carbonate) reacts with dilute hydrochloric acid.
Answer:
When Sodium bicarbonate (Sodium hydrogen carbonate) reacts with dilute hydrochloric acid, it gives sodium chloride, water and carbon dioxide gas is liberated.

Question 4.
Bleaching powder is exposed to air.
Answer:
When bleaching powder is exposed to air, it undergoes slow decomposition due to the carbon dioxide in air and chlorine gas is released.

Question 5.
When dry slaked lime reacts with chlorine gas.
Answer:
When dry slaked lime reacts with chlorine gas, it gives bleaching powder.

Question 6.
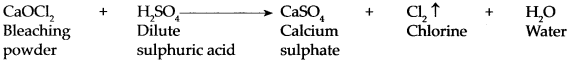
Bleaching powder reacts with dilute sulphuric acid.
Answer:
Dilute sulphuric acid reacts rapidly with bleaching powder to form calcium sulphate and release chlorine gas completely.
Question 7.
Bleaching powder reacts with dilute hydrochloric acid.
Answer:
Dilute hydrochloric acid reacts rapidly with bleaching powder to form Calcium chloride and release chlorine gas completely.
Question 8.
Sodium carbonate reacts with magnesium chloride.
Answer:
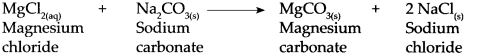
When Sodium carbonate reacts with magnesium chloride, it forms insoluble magnesium carbonate and sodium chloride. This reaction is used to make hard water soft.
Question 9.
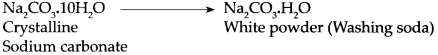
Crystalline sodium carbonate is exposed to air.
Answer:
When crystalline sodium carbonate is exposed to air it loses its water of crystallization readily and a white powder is obtained. This powder is called washing soda.
Question 10.
Sodium carbonate reacts with sulphuric acid.
Answer:
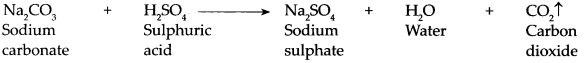
When sodium carbonate reacts with sulphuric acid it forms sodium sulphate, water and carbon dioxide gas is liberated.
Distinguish between;
Question 1.
Washing soda and Baking soda
Answer:
| Washing soda | Baking soda |
| (i) Chemical name is sodium carbonate. | (i) Chemical name is sodium bicarbonate or sodium hydrogen carbonate. |
| (ii) Its molecular formula is Na 2 CO 3 .10H 2 O | (ii) Its molecular formula is NaHCO 3 |
| (iii) It is a crystalline substance. | (iii) It is an amorphous powder. |
| (iv) It is used in manufacturing soaps and detergents. | (iv) It is used in bakery for making cakes and bread lighter and spongy. |
Question 2.
Bathing soap and Washing soap
Answer:
| Bathing soap | Washing soap |
| (i) High-grade fats and oils are used as the raw material. | (i) Low-grade fats and oils are used as the raw material. |
| (ii) Expensive perfumes added. | (ii) Cheaper perfumes added. |
| (iii) No free alkali content present to prevent injuries to skin. | (iii) Free alkali present for cleaning action. |
Answer the following:
Question 1.
Give the properties and uses of Sodium bicarbonate.
Answer:
Properties and uses of sodium bicarbonate
- (i) NaHCO 2 reacts with moist litmus paper and red litmus turns blue which means that it is basic in nature.
- (ii) It is used to make bread, cake, dhokla.
- (iii) Being basic in nature it is used to reduce acidity in the stomach.
- (iv) NaHCO 3 is used to make the active substance CO 2 in the fire extinguisher.
- (v) Baking soda is used to clean an oven.
Question 2.
Give the properties and uses of washing soda.
Answer:
Properties and uses of washing soda :
- Washing soda is a whitish and odourless powder at room temperature.
- Litmus has a blue colour in its aqueous solution.
- It is hygroscopic, that is, it absorbs moisture if left exposed to air.
- It is used mainly for washing clothes.
- Sodium carbonate is used in the glass and paper industry and also in refining of petrol.
Question 3.
Name some substances in our daily use which contain water of crystallization.
Answer:
- Alum (Potash alum – K 2 SO 4 .Al 2 (SO 4 ) 3 .24H 2 O)
- Borax (Na 2 B 4 O 7 .10H 2 O)
- Epsom salt (Magnesium sulphate MgSO 4 .7H 2 O)
- Barium chloride (BaCl 2 .2H 2 O)
- Sodium sulphate (Glauber’s salt Na 2 SO 4 .10H 2 O)
Question 4.
Give the uses of radioactive isotopes in the measurement of thickness, density and level.
Answer:
Measurement of thickness, density and level:
- It is necessary to maintain the required thickness in the manufacture of aluminium, plastic, iron sheets of differing thickness.
- In the manufacturing process, a radioactive substance is placed on one side and an instrument to measure radiation on the other.
- The radiation read by the measuring instrument varies with the thickness of the sheet.
- Material inside a packing can also be examined by the same technique.
Question 5.
Give the uses of radioactive isotopes in Luminescent paint and radioluminescence.
Answer:
Luminescent paint and radioluminescence:
- The radioactive substances radium, promethium, tritium with some phosphour are used to make certain objects visible in the dark, for example, the hands of a clock, and certain other objects.
- Krypton-85 is used in HID (High Intensity Discharge) lamps while promethium-147 is used in portable X-ray units as the source of beta rays.
Question 6.
Give the uses of radioactive isotopes in Field of agriculture.
Answer:
Field of agriculture:
- The genes and chromosomes that give seeds properties like fast growth, higher productivity, etc. can be modified by means of radiation.
- The radioactive isotope cobalt-60 is used for food preservation.
- Onions, potatoes are irradiated with gamma rays from cobalt-60 to prevent their sprouting.
- Strontium-90 is used as a tracer in the research on various crops.
Question 7.
Give the uses of dyes.
Answer:
Uses of dyes :
- They are used for colouring cloth and hair.
- Fluorescent colours are used to make street boards that are visible at night.
- Dyes are used to polish leather shoes, purses and chappals.
Question 8.
What are the adverse effects of dyes?
Answer:
Adverse effects dyes :
- Dyeing hair can have adverse effects like hair fall, damage to hair texture, burning of skin, adverse effect on eyes, etc.
- Lipstick contains a dye named carmine. It does not affect lips but causes stomach disorders.
- Excessive use of plants for making natural dyes results in deterioration of the environment.
Question 9.
Give the uses of teflon.
Answer:
Uses of teflon :
- Teflon is a poor conductor of electricity. Therefore, Teflon clad wires and parts are used in high technology electronic instruments.
- It is used for making non-stick kitchenware.
- The coloured metal sheets of two-wheelers and four-wheelers are given a Teflon coating to protect them from damage due to high temperature and rain.
Question 10.
What is ceramic? Give examples.
Answer:
- Ceramic is a heat resistant substance formed by kneading an inorganic substance in water and then shaping it and hardening it by heating.
- Pots made by a potter, Mangalore roofing tiles, construction bricks, pottery, terracotta articles are some examples of common ceramic articles that we see around.
Question 11.
Give the uses of ceramics.
Answer:
- Ceramics can withstand high temperatures without decomposing. Ceramic is brittle, water-resistant and an electrical insulator.
- Therefore, it is used in electrical instruments, for coating the interior of a kiln, the outer surfaces of ships and blades of jet engines.
- A certain type of ceramic tiles are fixed on the outer layer of a space shuttle. Some types of ceramics are used as superconductors.
Question 12.
What is brine? What happens when ^th of this solution is evaporated?
Answer:
- The 25% aqueous solution of salt is called saturated brine.
- When th of this solution is evaporated the dissolved salt gets crystallized and salt gets separated from the solution.
Question 13.
What is baking soda? Give its chemical name and molecular formula.
Answer:
- Baking soda is a white non-crystalline powder.
- Its chemical name is sodium hydrogen carbonate or sodium bicarbonate and its molecular formula is NaHC03.
Question 14.
Give two uses of Blue vitriol.
Answer:
- Blue vitriol is used in the blood test for diagnosing anaemia.
- Bordeaux mixture which is a mixture of slaked lime and blue vitriol is used as a fungicide on fruits like grapes and musk melon.
Question 15.
What is radioactivity and what are radioactive substances?
Answer:
- Elements with a high atomic number such as uranium, thorium, radium have a property of spontaneously emitting invisible, highly penetrating and high energy radiation. This property is called radioactivity.
- A substance having this property is called a radioactive substance.
Question 16.
What can you say about the uses of radioactive isotopes?
Answer:
- It is a misconception that radioactive elements are used only for making an atom bomb.
- Radioactive isotopes are used in various fields such as scientific research, agriculture, industry, medicine, etc.
- Radioactive substances are used in two ways.
- By using the radiation alone.
- By using the radioactive element itself.
Question 17.
Give the uses of radioactive isotopes in Radiography.
Answer:
- Internal cracks and voids in cast iron articles and iron solder can be detected with the help of gamma rays.
- For this purpose, isotopes like cobalt-60, iridium-192 are used in the radiography camera.
- This technique is used for detecting flaws in metalwork.
Question 18.
Give the uses of radioactive isotopes in ceramic articles.
Answer:
- Luminous colours are used to decorate ceramic tiles, utensils, plates, etc.
- Earlier uranium oxide was used in these paints.
Question 19.
What is a dye?
Answer:
The coloured substance which on applying to an article, imparts that colour to the article, is called a dye
Question 20.
What is done to fix a dye on the cloth ?
Answer:
- A mordant is to be used to fix the colour after dyeing a cloth.
- A mordant is a chemical binding agent that adheres well to both the fibers and the dye.
Question 21.
What is Teflon? Give its chemical name.
Answer:
- Teflon is the polymer of tetrafluoroethylene.
- Its chemical name is polytetrafluoroethylene (C 2 F 4 ) n
Question 22.
What is powder coating? Why is it done?
Answer:
- Powder coating is a method of applying a layer harder than paint on the surface of plastic, medium density fibre (MDF).
- Board and iron object to make them attractive, durable and to prevent rustihg respectively.
Question 23.
Give the properties of ceramics.
Answer:
- Ceramics can withstand high temperatures without decomposing.
- Ceramic is brittle, water resistant and an electrical insulator.
Question 24.
How is bone china made? How is it different from porcelain?
Answer:
- Bone china is made by adding some ash of animal bones in the mixture of china clay, feldspar and fine silica while making porcelain.
- This ceramic is harder than porcelain.
Question 25.
What compounds are used instead of clay for making advanced ceramic?
Answer:
Oxides like Alumina (Al
2
O
3
), Zirconia (ZrO
2
) Silica (SiO
2
) and some other compounds like silicon carbide (SiC), boron carbide (B
4
C) are used instead of clay for making advanced ceramic.
Question 26.
What is sintering?
Answer:
- Advanced ceramics requires a temperature of 1600 to 1800 °C and an oxygen-free atmosphere for firing.
- This process is called sintering.
Write short notes on :
Question 1.
Chernobyl disaster.
Answer:
- On 26th April 1986, the graphite reactor in the Chernobyl atomic power plant exploded, and suddenly the radioactive isotopes and radiation came out.
- Due to this episode, radioactive isotopes entered the human body through water and land and caused genetic disorders.
- These got carried further into the next generation.
- Thyroid disorders increased in children as well as adults.
- As a result, the incidence of throat diseases is greater there than in other places.
Question 2.
Food colours.
Answer:
- Food colours are mixed in most soft drinks and foodstuffs available in the market.
- These food colours are in the form of powders, gels and pastes.
- Food colours are used in domestic as well as commercial products.
- Certain colours and essences are added to ice cream, ice candies, sauces, fruit juices, cold drinks, pickles, jams and jellies.
- Food colours are often found to be added to packaged meat (chicken, mutton), chilli powder, turmeric, sweets and other similar substances so as to give them a good colour.
- Food colours are natural as well as artificial.
- The food colours prepared from seeds, beetroot, flowers and fruit concentrate are natural.
- Tetrazene, sunset yellow are artificial food colours used extensively.
- However, over-consumption of artificial food colours can be detrimental to health. Therefore, usage of natural food colours is always good.
Question 3.
Anodizing.
Answer:
- A protective layer is formed naturally on the surface of aluminium metal by reaction with oxygen in air.
- In the anodizing process, this layer can be made of the desired thickness.
- Anodizing is done by electrolysis.
- Dilute sulphuric acid is taken in the electrolytic cell and the aluminium article is dipped in it as the anode.
- When an electric current is passed, hydrogen gas is released at the cathode and oxygen gas at the anode.
- A reaction with oxygen occurs and a layer of hydrated aluminium oxide is formed on the anode, i.e. the aluminium article.
- This layer can be made attractive by adding colour in the cell during electrolysis.
Answer the following questions:
Question 1.
What are salts? Give their source.
Answer:
- The ionic compounds which do not contain H+ and OH ions and contain only one kind of cation and anion are called simple salts.
- For example, Na 2 SO 4 , K 3 PO 4 , CaCl 2 .
- The sea is said to be a rich source of salts of various elements such as chlorine, sodium, magnesium, potassium, calcium, bromine.
-
The important salts found in sea water are:
(a) Sodium chloride
(b) Magnesium chloride
(c) Magnesium sulphate
(d) Potassium chloride
(e) Calcium carbonate
(f) Magnesium bromide
Question 2.
Which are the three kinds of salts depending on their pH value? How are they formed?
Answer:
The three kinds of salts depending on their pH value are:
Neutral salts:
Salt is neutral when its pH value is 7. Such a salt is made from a strong acid and a strong base.
Acidic salts:
The pH value of a salt made from a strong acid and a weak base is less than 7 and it is acidic.
Basic salts:
The pH value of a salt made from a weak acid and strong base is more than 7 and it is basic.
Question 3.
Which is the most used salt? What is its chemical name? How is it formed?
Answer:
- Table salt, or common salt, which gives a salty taste to food, is the most used of all salts.
- Its chemical name is Sodium chloride.
- Sodium chloride is formed by a neutralization reaction between sodium hydroxide and hydrochloric acid.
- Chemical reaction
- This is a neutral salt and the pH value of its aqueous solution is 7.
Question 4.
How is bleaching powder obtained?
Answer:
- Bleaching powder is obtained by the reaction of chlorine gas with slaked lime.
- Chemical reaction
Question 5.
How is hard water from well converted to soft water?
Answer:
- The hard water from a well or a bore-well becomes soft on adding washing soda and we come to know this from the lather formed on it.
- The hardness of water is due to the presence of chlorides and sulphates of calcium and magnesium in it.
- Na 2 CO 3 is added to it to soften such water and make it suitable for use.
- The reaction with Na 2 CO 3 causes the formation of insoluble carbonate salts of magnesium and calcium.
-
Chemical reaction
MgCl 2(aq) + Na 2 CO 3(s) → MgCO 3(s) + 2NaCl (s) Magnesium Sodium Magnesium Sodium chloride carbonate carbonate chloride
Question 6.
How is washing soda obtained from sodium carbonate?
Answer:
- Sodium carbonate is a water soluble salt of sodium.
- Crystalline sodium carbonate, on keeping, loses its water of crystallization readily and a white powder is obtained.
- This powder is called washing soda.
- Chemical reaction
Question 7.
How is soap prepared? Why it does not form lather with hard water?
Answer:
- When oil or animal fat is boiled with an aqueous solution of sodium or potassium hydroxide, sodium or potassium salts of carboxylic acids (fatty acids) are formed.
- These salts are called soap.
- When soap is mixed with hard water, calcium and magnesium salts of fatty acids are formed.
- These being water insoluble, they form a precipitate and that is why lather is not formed.
Question 8.
What are the harmful effects of artificial food colours?
Answer:
- Food colours added to pickles, jams and sauces contain small quantities of lead and mercury. These can be harmful for those who consume these products on a regular basis.
- Diseases like ADHD (Attention Deficit Hyperactivity Disorder) can affect children due to excessive consumption of foods with added food colours.
Question 9.
Which is the main source of colour for preparing a natural dye?
Answer:
- Plants are the main source of colour for preparing a natural dye.
- Roots, leaves, flowers, bark, fruits, seeds, fungus and pistils are used for making dyes.
- In Kashmir a very good dye is made from saffron, which is used to dye fibers from which saris, shawls and dresses are made.
- These are very costly. The use of henna leaves to colour hair is safe for health.
Question 10.
How are artificial colours harmful to us?
Answer:
- We regularly use artificial colours on Rang Panchami.
- The red colour used on Rang Panchami is very dangerous.
- It contains a high proportion of mercury in it.
- This poses risks like blindness, skin cancer, asthma, itching of the skin, permanent blocking of sweat pores, etc.
- Therefore, it is necessary that artificial colours are used cautiously.
Question 11.
What are the harmful effects of deodorants?
Answer:
- Aluminium – Zirconium compounds are the most harmful chemicals in deodorants. Disorders like headache, asthma, respiratory disorders, heart disease are likely to occur without our knowledge.
- There is a possibility of various skin disorders and also skin cancer due to the aluminium chlorohydrates.
Question 12.
How is a ceramic article made?
Answer:
- When clay is kneaded in water, shaped and then fired in a kiln at a temperature of 1000 to 1150 °C, a porous ceramic is formed.
- To overcome the porosity, the fired object is covered with finely ground glass powder suspended in water (glaze) and is then fired again. As a result, the surface of the ceramic becomes shiny and its porosity disappears.
Question 13.
What is porcelain? How is it made?
Answer:
- Porcelain is a hard, translucent and white coloured ceramic. It is made by using the white clay called kaolin, found in China. Glass, granite and the mineral feldspar is mixed with kaolin and kneaded with water.
- The resulting mixture is shaped and fired in a kiln at a temperature of 1200 to 1450 °C. On firing again after glazing, beautiful articles of porcelain are obtained.
Answer the following questions:
Question 1.
What are the constituents of baking powder? Where is the baking powder used?
Answer:
- Baking powder consist of 30% sodium bicarbonate, 5-12% monocalcium phosphate and 21-26% sodium aluminium sulphate.
- Baking powder is a leavening agent and is used for increasing the volume and lightening the texture of baked goods.
Question 2.
Take a piece of coloured cloth. Put some saturated solution of bleaching powder on a small part and observe what changes take place in the colour of the cloth.
Answer:
When bleaching powder is added to a piece of coloured cloth, the fabric looses its colour due to the oxidising reaction of the chlorine present in the bleaching powder.
Answer in detail:
Question 1.
Give the properties and uses of Sodium chloride.
Answer:
Properties and uses of sodium chloride:
- Common salt is a colourless and crystalline ionic compound. There is no water of crystallization in its crystalline structure.
- It is a neutral salt, salty in taste.
- This compound is used for the production of salts like Na 2 CO 3 , NaHCO 3 .
- When an electric current is passed through a saturated solution of sodium chloride (brine), it is electrolysed and hydrogen gas is released at the cathode while chlorine gas is released at the anode.
-
This method is used for production of chlorine gas. In this method an important basic compound NaOH is formed in the cell.
2NaCl + 2H 2 O → 2NaOH + Cl 2 ↑ + H 2 ↑ - When salt is heated to a high temperature (about 800 °C), it melts. This is called the fused state of the salt.
- When fused salt is electrolysed, chlorine gas is released at the anode and liquid sodium metal, at the cathode.
Question 2.
Give the properties and uses of Bleaching Powder.
Answer:
Properties and uses bleaching powder:
- Bleaching powder is a yellowish-white coloured solid substance.
- Its chemical name is Calcium oxychloride.
- It has a strong odour of chlorine gas.
- It is used for disinfection of drinking water at the water works and the water in the swimming pool.
- It is used for bleaching of cloth.
- It is used for disinfection by the road side and garbage sites.
-
Dilute sulphuric acid and dilute hydrochloric acid react rapidly with bleaching powder to release chlorine gas completely.
CaOCl 2 + H 2 SO 4 → CaSO 4 + Cl 2 ↑ + H 2 O CaOCl 2 + 2HCl → CaCl 2 + Cl 2 ↑ + H 2 O -
Calcium oxychloride reacts slowly with carbon dioxide to form calcium carbonate and chlorine.
CaOCl 2 + CO 2 → CaCOs + Cl 2 ↑
Question 3.
Explain with neat labelled diagram the nature of radioactive radiation.
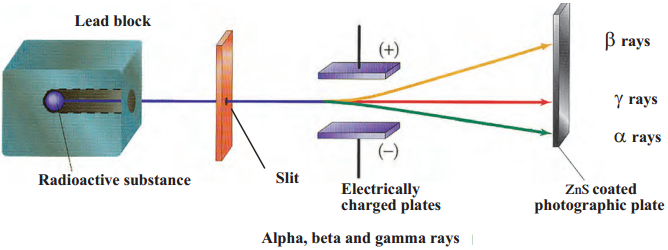
Answer:
- When radioactive radiations are allowed to pass through two oppositely charged plates they get separated. This method was introduced by Rutherford in 1902.
- Rutherford and Willard studied the radiation emitted by radioactive substances.
- For this purpose, the rays were allowed to pass through an electrical field and a photographic plate was held in their path.
- It was found that the radiation was divided into three types.
- One type of radiation deviated slightly towards the negatively charged plate, while the second type of radiation deviated substantially towards the positively charged plate.
- However, the third type of radiation did not deviate at all in the electrical field.
- The rays which deviated slightly toward negatively charged plate are called alpha rays.
- Those which deviate substantially towards the positively charged plate are called beta rays and those which did not deviate at all are called gamma rays.
Question 4.
Give the characteristics of alpha, beta and gamma rays.
Answer:
Characteristics of alpha, beta and gamma rays are as given in the table.
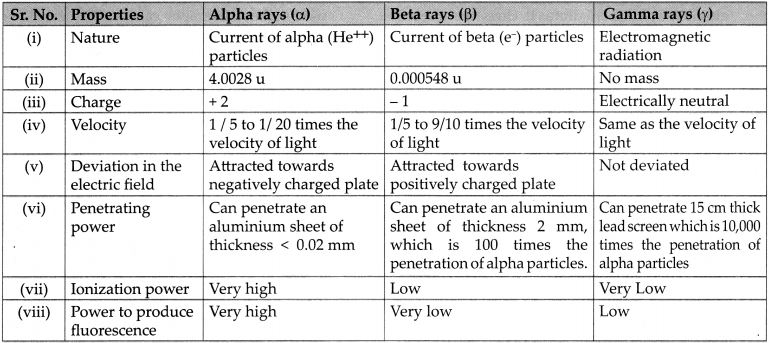
Question 5.
Give the uses of radioactive isotopes in Medical science.
Answer:
Medical science:
- Polycythemia: The red blood cell count increases in the disease polycythemia. Phosphorus- 32 is used in its treatment.
- Bone cancer: Strontium-89, strontium-90, samarium-153 and radium-223 are used in the treatment of bone cancer.
- Hyperthyroidism: Enlargement of thyroid gland, weight loss in spite of appetite, insomnia are the symptoms of hyperthyroidism.
- It occurs due to overproduction of hormones by the thyroid gland. Iodine-123 is used in the treatment of hyperthyroidism.
- Tumour detection: Boron-10, iodine-131, cobalt-60 are used in the treatment of brain tumour, while arsenic-74 is used in detection of small tumours in the body.
Question 6.
What are the hazards of radioactive substances and radiation?
Answer:
- The central nervous system is affected by radioactive radiations.
- Hereditary defects are generated by bombardment of radiation on D.N.A in the body.
- Radioactive radiation can penetrate the skin, and causes diseases like skin cancer, leukemia.
- The radiative pollutants created due to explosions enter the body through air and it is difficult to control them.
- The radioactive pollutants released in the sea enter the bodies of fishes and through them enter the human body.
- The radioactive paint on the watch can cause cancer.
- The radioactive isotopes strontium-90 can enter the body through plants, fruits, flowers, cereals, milk, etc. and cause diseases like bone cancer, leukemia.
Question 7.
Why are deodorant used? Give their types and content.
Answer:
- Body odour is caused by the bacterial decomposition of the sweat. A deodorant is used to prevent this odour.
- Deodorants contain parabens (methyl, ethyl, propyl, benzyl and butyl) and also alcohol in large proportions. Aluminium compounds and silica are also used.
-
Types of deodorants are :
(a) Ordinary deo: It contains a smaller proportion of aluminium. It decreases the odour of the sweat.
(b) Antiperspirant deo : This decreases the extent of sweating. It contains about 15% of aluminium chlorohydrate. It clogs the sweat pores on the skin.
(c) Clinical deo : Some people sweat heavily and it has harmful effects on the skin. Clinical deo is meant for such people. It contains 20 to 25% aluminium. It is used during the night.
Question 8.
Explain how powder coating is done.
Answer:
- Powder coating is a method of applying a layer harder than paint on the surface of an iron object to prevent rusting.
- In this method, a polymer resin, a pigment and some other ingredients are melt, mixed, cooled and ground into a uniform powder.
- This powder is sprayed on the polished metal surface by electrostatic spray deposition (ESD).
- In this method, the particles of the powder are given an electrostatic charge due to which a uniform layer of the powder sticks to the metal surface.
- Then the object is heated in the oven along with the coating.
- A chemical reaction occurs in the layer, resulting in the formation of long cross-linked polymeric chains.
- This powder coating is highly durable, hard and attractive.
- Powder coating can be done on plastic and medium density fibre (MDF) board in day to day use as well.
