Balbharti Maharashtra State Board 12th Chemistry Textbook Solutions Chapter 10 Halogen Derivatives Textbook Exercise Questions and Answers.
Halogen Derivatives Class 12 Exercise Question Answers Solutions Maharashtra Board
Class 12 Chemistry Chapter 10 Exercise Solutions Maharashtra Board
Chemistry Class 12 Chapter 10 Exercise Solutions
1. Choose the most correct option.
Question i.
The correct order of increasing reactivity of C-X bond towards nucleophile in the following compounds is
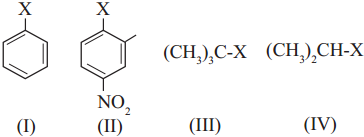
a. I < II < III < IV
b. II < I < III < IV
c. III < IV < II < I
d. IV < III < I < II
Answer:
(d) IV < III < I < II

Question ii.
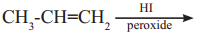
The major product of the above reaction is,
Answer:
(c)
Question iii.
Which of the following is likely to undergo racemization during alkaline hydrolysis?
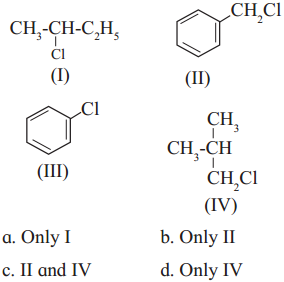
Answer:
(a) Only I
Question iv.
The best method for preparation of alkyl fluorides is
a. Finkelstein reaction
b. Swartz reaction
c. Free radical fluorination
d. Sandmeyer’s reaction
Answer:
b. Swartz reaction
Question v.
Identify the chiral molecule from the following.
a. 1-Bromobutane
b. 1,1- Dibromobutane
c. 2,3- Dibromobutane
d. 2-Bromobutane
Answer:
(d) 2-Bromobutane
Question vi.
An alkyl chloride on Wurtz reaction gives 2,2,5,5-tetramethylhexane. The same alkyl chloride on reduction with zinc-copper couple in alchol give hydrocarbon with molecular formula C
5
H
12
. What is the structure of alkyl chloride
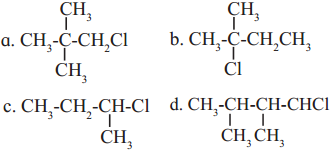
Answer:
(a)
Question vii.
Butanenitrile may be prepared by heating
a. propanol with KCN
b. butanol with KCN
c. n-butyl chloride with KCN
d. n-propyl chloride with KCN
Answer:
(d) n-propyl chloride with KCN
Question viii.
Choose the compound from the following that will react fastest by S
N
1 mechanism.
a. 1-iodobutane
b. 1-iodopropane
c. 2-iodo-2 methylbutane
d. 2-iodo-3-methylbutane
Answer:
(c) 2-iodo-2 methylbutane

Question ix.
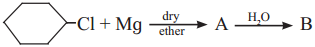
The product ‘B’ in the above reaction sequence is,
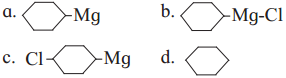
Answer:
(d)
Question x.
Which of the following is used as source of dichlorocarbene
a. tetrachloromethane
b. chloroform
c. iodoform
d. DDT
Answer:
(b) chloroform
2. Do as directed.
Question i.
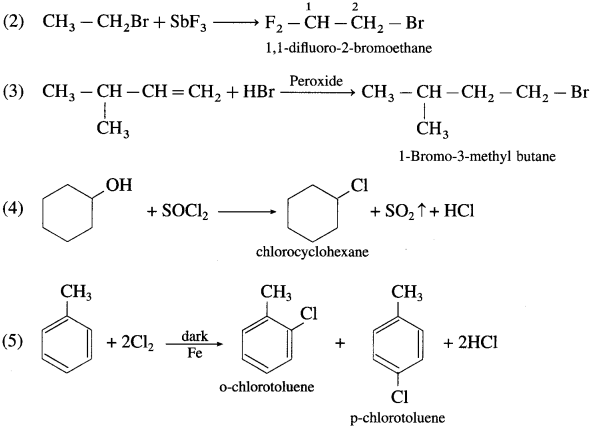
Write IUPAC name of the following compounds
Answer:
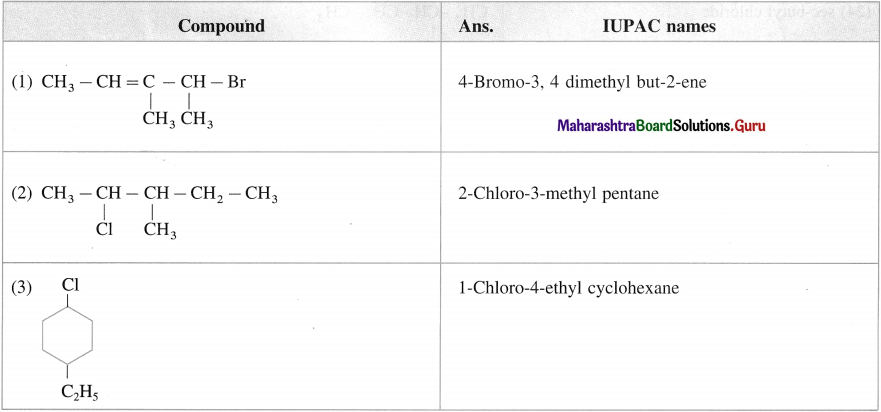

Question ii.
Write structure and IUPAC name of the major product in each of the following reaction.
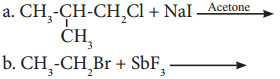
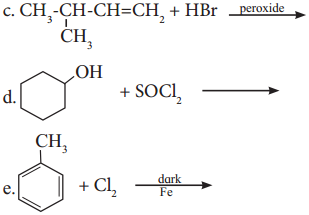
Answer:
Structure and IUPAC name
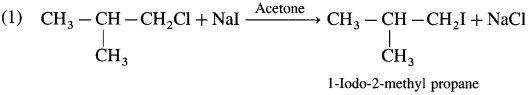

Question iii.
Identify chiral molecule/s from the following.
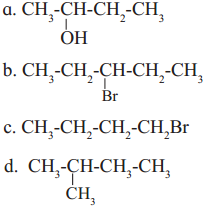
Answer:
Chiral molecule
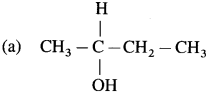
Question iv.
Which one compound from the following pairs would undergo S
N
2 faster from the?
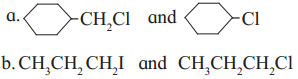
Answer:
(1) Sincey
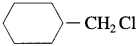 is a primary halide it undergoes S
N
2 reaction faster than
is a primary halide it undergoes S
N
2 reaction faster than
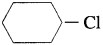 .
.
(2) Since iodine is a better leaving group than chloride, 1-iodo propane (CH
3
CH
2
CH
2
I) undergoes S
N
2 reaction faster than l-chloropropane (CH
3
CH
2
CH
2
CI).
Question v.
Complete the following reactions giving major product.
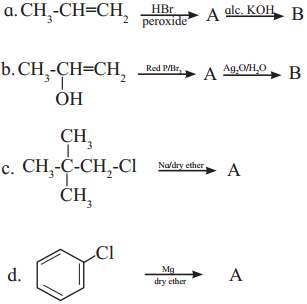
Answer:
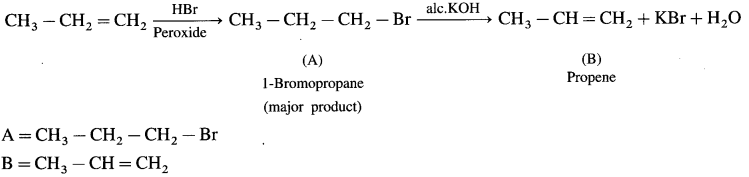
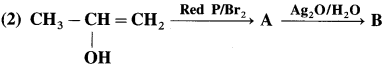
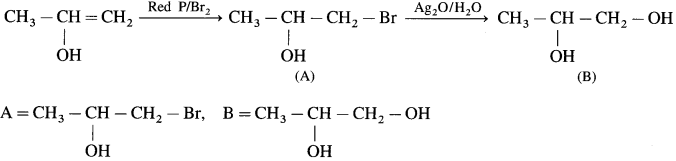
Answer:
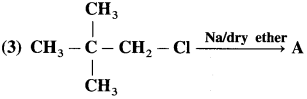
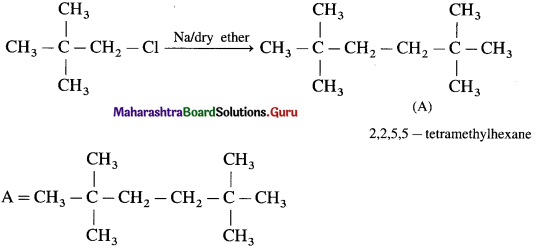
Answer:

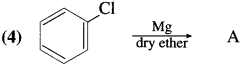
Answer:
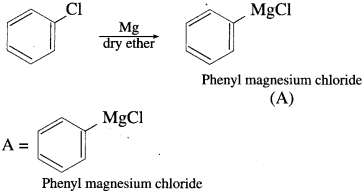
Question vi.
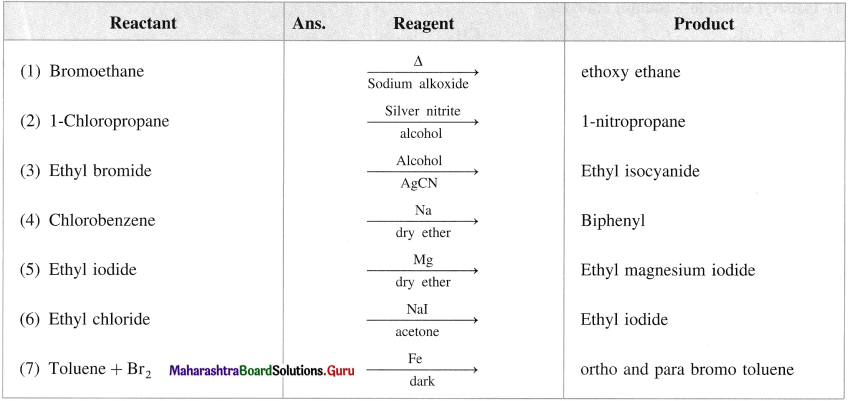
Name the reagent used to bring about the following conversions.
a. Bromoethane to ethoxyethane
b. 1-Chloropropane to 1 nitropropane
c. Ethyl bromide to ethyl isocyanide
d. Chlorobenzene to biphenyl
Answer:
Question vii.
Arrange the following in the increase order of boiling points
a. 1-Bromopropane
b. 2- Bromopropane
c. 1- Bromobutane
d. 1-Bromo-2-methylpropane
Answer:
l-Bromo-2-methylpropane, 2-Bromopropane, 1-Bromopropane, 1-Bromo butane
Question viii.
Match the pairs.
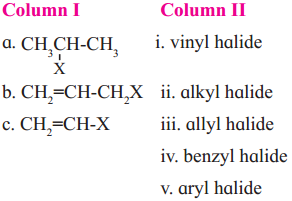
Answer:
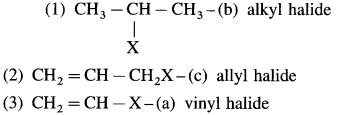

3. Give reasons
Question i.
Haloarenes are less reactive than haloalkanes.
Answer:
Haloarenes (Aryl halides) are less reactive than (alkyl halides) haloalkanes due to the following reasons :
(1) Resonance effect : In haloarenes, the electron pairs on halogen atom are in conjugation with 7r-electrons of the benzene ring. The delocalization of these electrons C-Cl bond acquires partial double bond character.
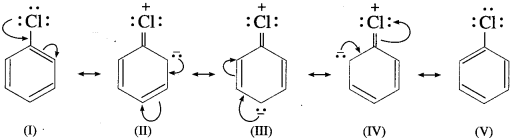
Due to partial double bond character of C-Cl bond in aryl halides, the bond cleavage in haloarene is difficult and are less reactive. On the other hand, in alkyl halides, carbon is attached to chlorine by a single bond and it can be easily broken.
(2) Aryl halides are stabilized by resonance but alkyl halides are not. Hence, the energy of activation for the displacement of halogen from aryl halides is much greater than that of alkyl halides.
(3) Different hybridization state of carbon atom in C-X bond :
(i) In alkyl halides, the carbon of C-X bond is sp
3
-hybridized with less 5-character and greater bond length of 178 pm, which requires less energy to break the C-X bond.
(ii) In aryl halides, the carbon of C-X bond is sp 3 -hybridized with more 5-character and shorter bond length which requires more energy to break C-X bond. Therefore, aryl halides are less reactive than alkyl halides.
(iii) Polarity of the C-X bond : In aryl halide C-X bond is less polar than in alkyl halides. Because sp 3 -hybrid carbon of C-X bond has less tendency to release electrons to the halogen than a sp 3 -hybrid carbon in alkyl halides. Thus halogen atom in aryl halides cannot be easily displaced by nucleophile.
(2) Aryl halides are extremely less reactive towards nucleophilic substitution reactions.
Answer:
Aryl halides are extremely less reactive towards nucleophilic substitution reaction due to the following reasons : (1) Resonance effect : In haloarenes, the electron pairs on halogen atom are in conjugation with 7r-electrons of the benzene ring. The delocalization of these electrons C-Cl bond acquires partial double bond character.
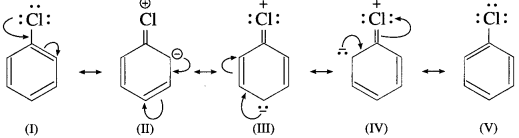
Due to partial double bond character of C-Cl bond in aryl halides, the bond cleavage in haloarene is difficult and are less reactive towards nucleophilic substitution.
(2) Sp 2 hybrid state of C : Different hybridization state of carbon atom in C-X bond : In aryl halides, the carbon of C-X bond is sp 2 -hybridized with more 5-character and shorter bond length of 169 pm which requires more energy to break C-X bond. It is difficult to break a shorter bond than a longer bond, in alkyl chloride (bond length 178 pm) therefore, aryl halides are less reactive towards nucleophilic substitution reaction.
(3) Instability of phenyl cation : In aryl halides, the phenyl cation formed due to self ionisation will not be stabilized by resonance which rules out possibility of S N 1 mechanism. Also backside attack of nucleophile is blocked by the aromatic ring which rules out S N 2 mechanism. Thus cations are not formed and hence aryl halides do not undergo nucleophilic substitution reaction easily.
(4) As any halides are electron rich molecules due to the presence of re-bond, they repel electron rich nucleophilic, attack. Hence, aryl halides are less reactive towards nucleophilic substitution reactions. However, the presence of electron withdrawing groups at o/p position activates the halogen of aryl halides towards substitution.
(3) Aryl halides undergo electrophilic substitution reactions slowly.
Answer:
Aryl halides undergo electrophilic substitution reactions slowly and it can be explained as follows :

(1) Inductive effect : The strongly electronegative halogen atom withdraws the electrons from carbon, atom of the ring, hence aryl halides show reactivity towards electrophilic attack.
(2) Resonance effect : The resonating structures of aryl halides show increase in electron density at ortho and para position, hence it is o, p directing.
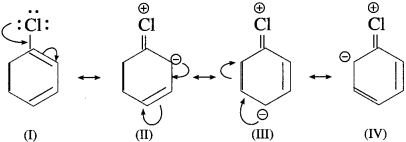
The inductive effect and resonance effect compete with each other. The inductive effect is stronger than resonance effect. The reactivity of aryl halides is controlled by stronger inductive effect and o, p orientation is controlled by weaker resonating effect.
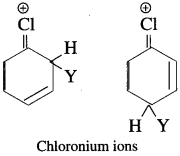
The attack of electrophile (Y) on haloarenes at ortho and para positions are more stable due to formation of chloronium ion. The chloronium ion formed is comparatively more stable than other hybrid structures of carbonium ion.
(4) Reactions involving Grignard reagent must be carried out under anhydrous condition.
Answer:
(1) Grignard reagent (R Mg X) is an organometallic compound. The carbon-magnesium bond is highly polar and magnesium halogen bond is in ionic in nature. Grignard reagent is highly reactive.
(2) The reactions of Grignard reagent are carried out in dry conditions because traces of moisture may spoil the reaction and Grignard reagent reacts with water to produce alkane. Hence, reactions involving Grignard reagent must be carried out under anhydrous condition.
(5) Alkyl halides are generally not prepared by free radical halogenation of alkane.
Answer:
(1) Free radical halogenation of alkane gives a mixture of all different possible Monohaloalkanes as well as polyhalogen alkanes.
(2) In this method, by changing the quantity of halogen the desired product can be made to predominate over the other
products. Hence, alkyl halides are generally not prepared by free radical halogenation of alkane.
Question ii.
Alkyl halides though polar are immiscible with water.
Answer:
(1) In alkyl halide, the halogen atom is more electronegative than carbon atom, the C – X bond is polar.
(2) Though alkyl halide is polar, it is insoluble in water because alkyl halide is not able to form hydrogen bonds with water. Attraction between alkyl halide molecule is stronger than attraction between alkyl halide and water.
(2) C-F bond in CH
3
F is the strongest bond and C-I bond in CH
3
I is the weakest bond. Explain.
Answer:
(1) Methyl fluoride (CH
3
F) is highly polar molecule and has the shortest C-F bond length (139 pm) and the strongest C-F bond due to greater overlap of orbitals of the same principal quantum number i.e., overlap of 2sp
3
orbital of carbon with 2pz orbital of fluorine.
(2) Methyl iodide (CH
3
I) is much less polar and has the longest (C-I) bond length (214 pm) and the weakest C-I bond due to poor overlap of 2sp
3
orbital carbon with 5pz orbital of iodine i.e., 2sp
3
orbital of carbon cannot penetrate into larger p-orbitals.
(3) The boiling point of alkyl iodide is higher than that of alkyl fluoride.
Answer:
For a given alkyl group, the boiling point increases with increasing atomic mass of the halogen, because magnitude of van der Waals force increases with increase in size and mass of halogen. Therefore, boiling point of alkyl iodide is higher than that of alkyl fluoride.
(4) The boiling point of isopropyl bromide is lower than that of it-propyl bromide.
Answer:
For isomeric alkyl halides (isopropyl bromide and n-propyl bromide), the boiling point decreases as the branching increases, surface area decreases on branching and van der Waals forces decrease, therefore, the boiling point of isopropyl bromide is lower than that of n-propyl bromide.
(5) p-Dichlorobenzene
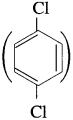 has mp. higher than those of o-and rn-isomers.
has mp. higher than those of o-and rn-isomers.
Answer:
p-Dichlorobenzene has higher melting point than those of o-and m-isomers. This is because of its symmetrical structure which can easily fits in crystal lattice. As a result intermolecular forces of attraction are stronger and therefore greater energy is required to overcome its lattice energy.

Question iii.
Reactions involving Grignard reagent must be carried out under anhydrous conditions.
Question iv.
Alkyl halides are generally not prepared by free radical halogenation of alkanes.
Answer:
(1) Direct fluorination of alkanes is highly exothermic, explosive and invariably leads to polyfluorination and decomposition of the alkanes. It is difficult to control the reaction.
(2) Direct iodination of alkanes is highly reversible and difficult to carry out.
(3) In direct chlorination and bromination, the reaction is not selective. It can lead to different isomeric monohalogenated alkanes (alkyl halides) as well as polyhalogenated alkanes.
Hence, halogenation of alkanes is not a good method of preparation of alkyl halides.
4. Distinguish between – S
N
1 and S
N
2 mechanism of substitution reaction ?
Answer:
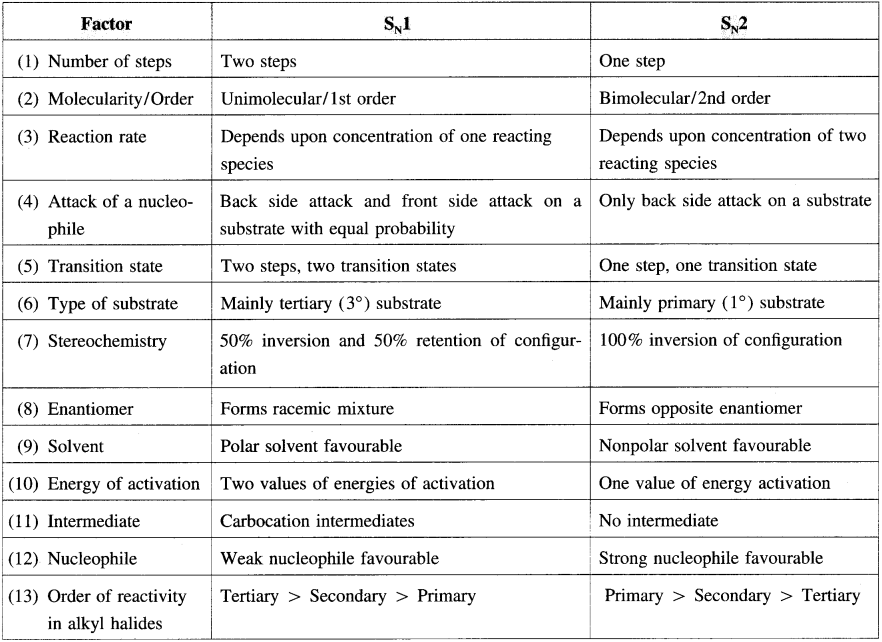
5. Explain – Optical isomerism in 2-chlorobutane.
Answer:
(1) 2-Chlorobutane contains an asymmetric.
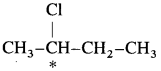 carbon atom (the starred carbon atom) which is attached to four different groups, i.e., ethyl (-CH
2
– CH
3
), methyl (CH
3
), chloro (Cl) and hydrogen (H) groups.
carbon atom (the starred carbon atom) which is attached to four different groups, i.e., ethyl (-CH
2
– CH
3
), methyl (CH
3
), chloro (Cl) and hydrogen (H) groups.
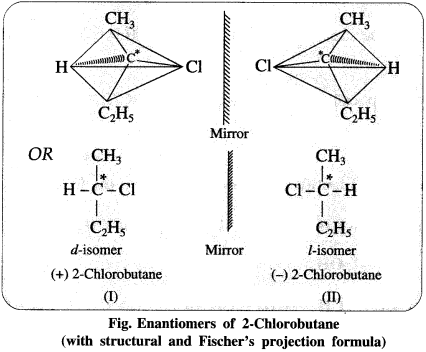
(2) Two different arrangements of these groups around the carbon atom are possible as shown in the figure. Hence, it exists as a pair of enanti¬omers. The two enantiomers are mirror images of each other and are not superimposable.
(3) One of the enantiomers will rotate the plane of plane-polarized light to the left hand side and is called the laevorotatory isomer (/-isomer). The other enantiomer will rotate the plane of plane-polarized light to the right hand side and is called the dextrorotatory isomer (d-isomer).
(4) Equimolar mixture of the d- and the 1-isomers is optically inactive and is called the racemic mixture or the racemate (dl-mixture). The optical inactivity of the racemic mixture is due to external compensation.

6. Convert the following.
Question i.
Propene to propan-1-ol
Answer:
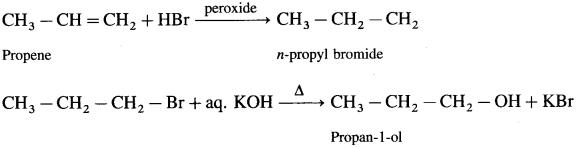
Question ii.
Benzyl alcohol to benzyl cyanide
Answer:
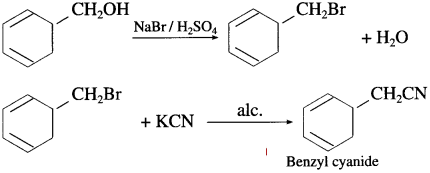
Question iii.
Ethanol to propane nitrile
Answer:
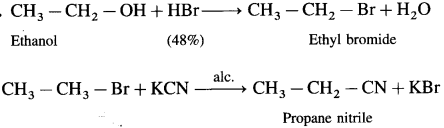
Question iv.
But-1-ene to n-butyl iodide
Answer:
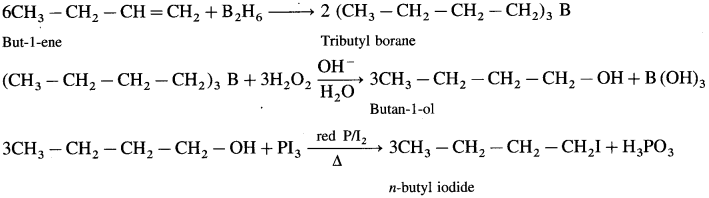
Question v.
2-Chloropropane to propan-1-ol
Answer:
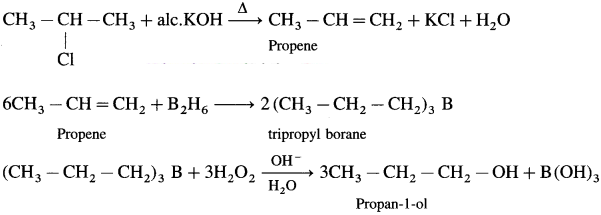
Question vi.
tert-Butyl bromide to isobutyl bromide
Answer:
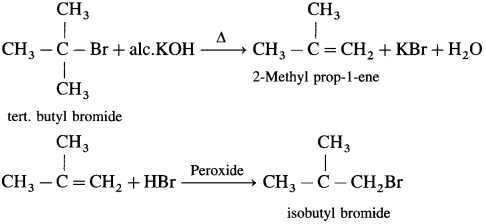

Question vii.
Aniline to chlorobenzene
Answer:
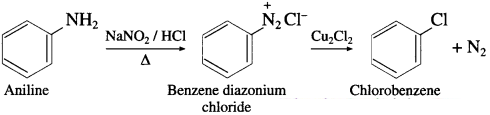
Question viii.
Propene to 1-nitropropane
Answer:
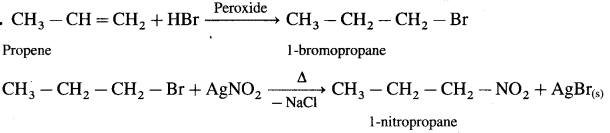
7. Answer the following
Question i.
HCl is added to a hydrocarbon ‘A’ (C
4
H
8
) to give a compound ‘B’ which on hydrolysis with aqueous alkali forms tertiary alcohol ‘C’ (C
4
H10O). Identify ‘A’ , ‘B’ and ‘C’.
Answer:
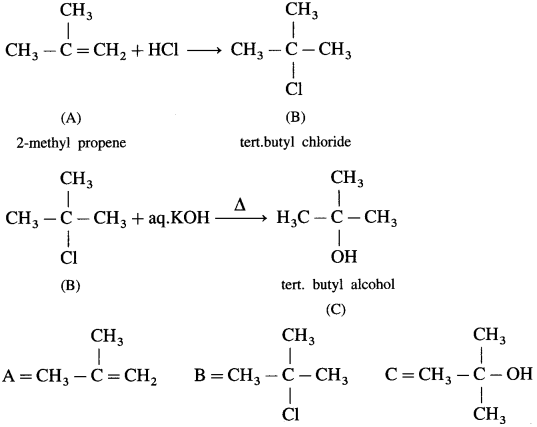
Question ii.
Complete the following reaction sequences by writing the structural formulae of the organic compounds ‘A’, ‘B’ and ‘C’.
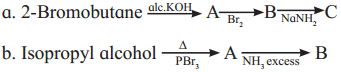
Answer:
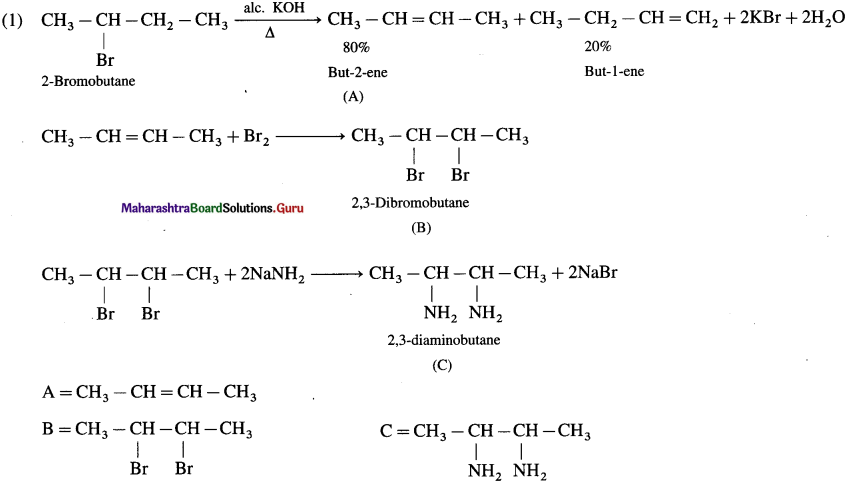
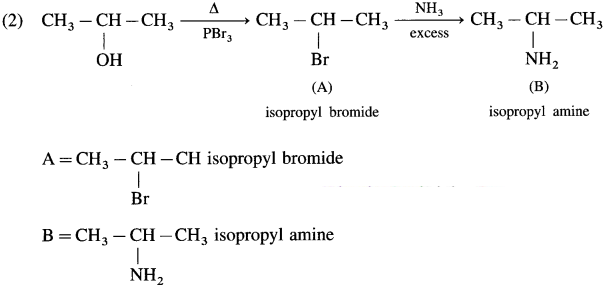

Question iii.
Observe the following and answer the questions given below.
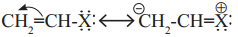
a. Name the type of halogen derivative
b. Comment on the bond length of C-X bond in it
c. Can react by S
N
1 mechanism? Justify your answer.
Answer:
a. Vinyl halide
b. C – X bond length shorter in vinyl halide than alkyl halide. Vinyl halide has partial double bond character due to resonance.
In vinyl halide, carbon is sp hybridised. The bond is shorter and stronger and the molecule is more stable.
c. Yes, It reacts by S N 1 mechanism. S N 1 mechanism involves formation of carbocation intermediate. The vinylic carbocation intermediate formed is resonance stabilized, hence S N 1 mechanism is favoured.
Activity :
1. Collect detailed information about Freons and their uses.
2. Collect information about DDT as a persistent pesticide.
Reference books
i. Organic chemistry by Morrison, Boyd, Bhattacharjee, 7
th
edition, Pearson
ii. Organic chemistry by Finar, Vol 1, 6
th
edition, Pearson
12th Chemistry Digest Chapter 9 Halogen Derivatives Intext Questions and Answers
Use your brain power….. (Textbook page 212)
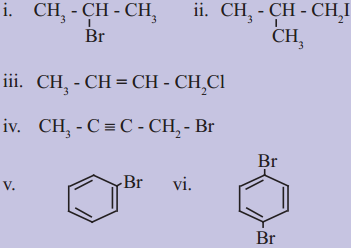
Question 1.
Write IUPAC names of the following:
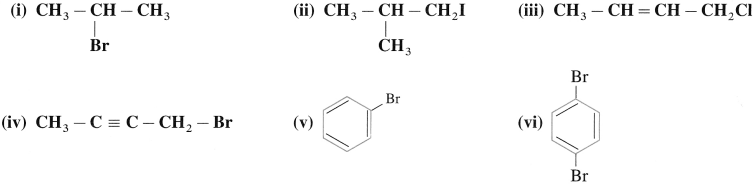
Answer:

Question 10.1 : (Textbook page 213)
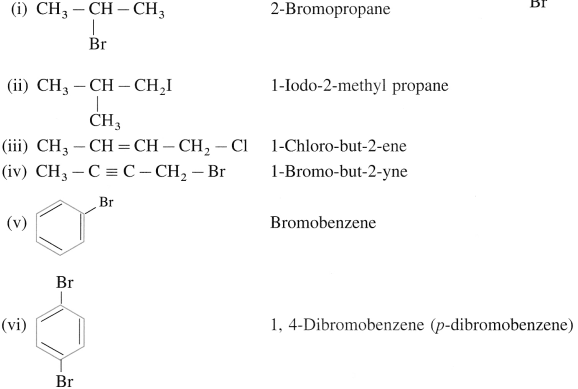
How will you obtain 1.bromo.1-methylcyclohexane from alkene? Write possible structures of alkene and the reaction involved.
Answer:

Use your brain power ….. (Textbook page 213)
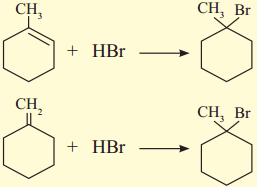
Question 1.
Rewrite the following reaction by filling the blanks:
Answer:
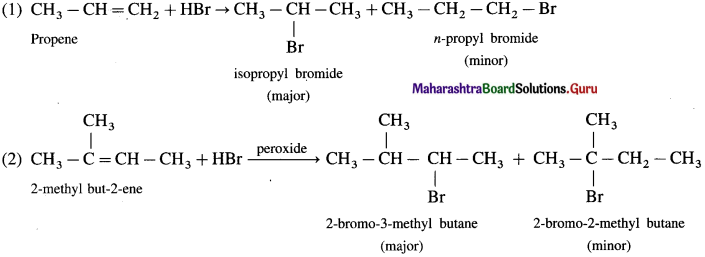
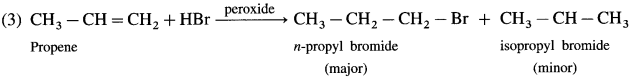

Question 10.2 : (Textbook page 216)
Arrange the following compounds in order of increasing boiling points : bromoform, chloromethane, dibromomethane, bromomethane.
Answer:
The comparative boiling points of halogen derivatives are mainly related with van der Waals forces of attraction which depend upon the molecular size. In the present case all the compounds contain only one carbon. Thus the molecular size depends upon the size of halogen and number of halogen atoms present.
Thus increasing order of boiling point is, CH 3 CI < CH 3 Br < CH 2 Br 2 < CHBr 3
Try this ….. (Textbook page 2016)
Question 1.
(1) Make a three-dimensional model of 2-chlorobutane.
(2) Make another model which is a mirror image of the first model.
(3) Try to superimpose the two models on each other.
(4) Do they superimpose on each other exactly ?
(5) Comment on whether the two models are identical or not.
Answer:
(1) (2) and (3)
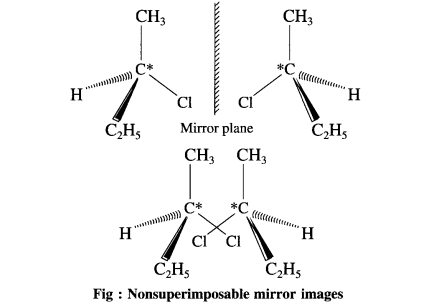
(4) Two models are non-superimposable mir ror images of each other called enantiomers.
(5) Two enantiomers are identical. Theyhave the same physical properties (such as melting points, boiling points, densities refractive index). They also have identical chemical properties. The magnitude of their optical rotation is equal but the sign of optical rotation is opposite.
Try this ….. (Textbook page 219)
Question 1.
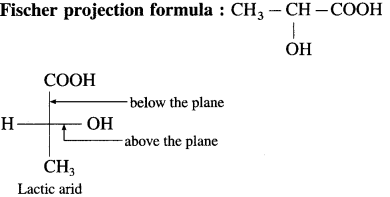
1. Draw structares of enantiomers of lactic acid
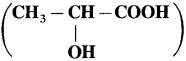 using Fischer projection formulae.
using Fischer projection formulae.
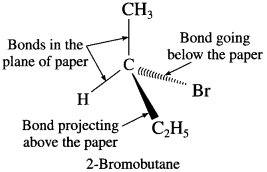
2. Draw structures of enantiomers of 2-bromobutane using wedge formula.
Answer:
(1)
(2) Wedge formula : 2-brornobutane

Can you tell? (Textbook page 220)
Question 1.
Alkyl halides, when treated with alcoholic solution of silver nitrite, give nitroalkanes whereas with sodium nitrite they give alkyl nitrites. Explain.
Answer:
Nitrite ion is an ambident nucleophile, which can attack through ‘O’ or ‘N’.
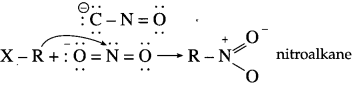
Both nitrogen and oxygen are capable of donating electron pair. C – N bond, being stronger than N – O bond, attack occurs through C atom from alkyl halide forming nitroalkane.
However, sodium nitrite (NaNO
2
) is an ionic compound and oxygen is free to donate pair of electrons. Hence, attack occurs through oxygen resulting in the formation of alkyl nitrite.
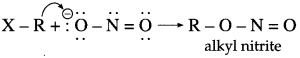
Use your brain power! (Textbook page 222)
Question 1.
Draw the Fischer projection formulae of two products obtained when compound (A) reacts with OHe by S
N
1 mechanis.
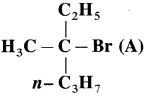
Answer:
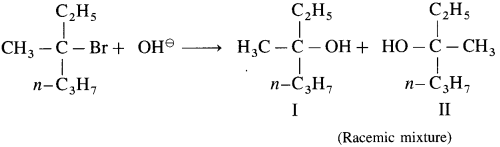
Question 2.
Draw the Fischer projection formula of the product formed when compound (B) reacts with OHΘ by S
N
2 mechanism.
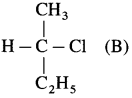
Answer:
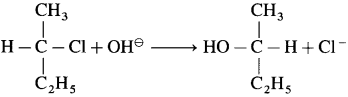
Question 10.4 : (Textbook page 223)
Allylic and benzylic halides show high reactivity towards the S
N
1 mechanism than other primary alkyl halides. Explain.
Answer:
In allylic and benzylic halide, the carbocation formed undergoes stabilization through the resonance. Hence, allylic and benzylic halides show high reactivity towards the S
N
1 reaction. The resonating structures are
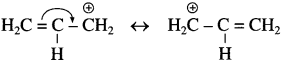
Resonance stabilization of allylic carbocation
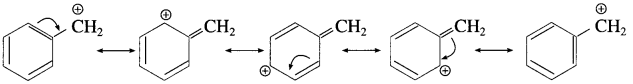
Resonance stabilization of benzylic carbocation

Question 10.5 : (Textbook page 224)
Which of the following two compounds would react faster by SN2 mechanism and Why?
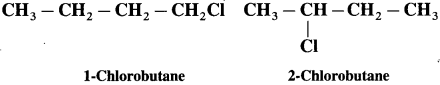
Answer :
In S
N
2 mechanism, a pentacoordinate T.S. is involved. The order of reactivity of alkyl halides towards S
N
2 mechanism is.
Primary > Secondary > Tertiary, (due to increasing crowding in T.S. from primary to tertiary halides.
1- Chlorobutane being primary halide will react faster by S
N
2 mechanism, than the secondary halide 2- chlorobutane.)
Can you tell? (Textbook page 227)
Question 1.
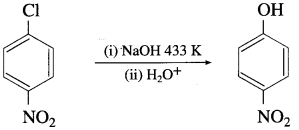
Conversion of chlorobenzene to phenol by aqueous sodium hydroxide requires a high temperature of about 623K and high pressure. Explain.
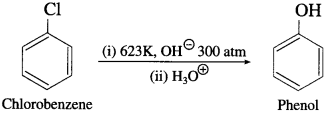
Answer:
Due to the partial double bond character in chlorobenzene, the bond cleavage in chlorobenzene is difficult and is less reactive. Hence, during the conversion of chlorobenzene to phenol by a question NaOH requires high temperature & high pressure.
